Abstract
Mitotic progression is regulated by ubiquitin E3 ligase complexes to carefully orchestrate eukaryotic cell division. Here, we show that a relatively new E3 ligase component belonging to the SCF (Skip-Cullin1-F-box protein) E3 ligase family, SCFFBXL2, impairs cell proliferation by mediating cyclin D3 polyubiquitination and degradation. Both cyclin D3 and FBXL2 colocalize within the centrosome. FBXL2 overexpression led to G2/M-phase arrest in transformed epithelia, resulting in the appearance of supernumerary centrosomes, tetraploidy and nuclei where condensed chromosomes are arranged on circular monopolar spindles typical of mitotic arrest. RNAi-mediated knockdown of cyclin D3 recapitulated effects of SCFFBXL2 expression. SCFFBXL2 impaired the ability of cyclin D3 to associate with centrosomal assembly proteins [Aurora A, polo-like kinase 4 (Plk4), CDK11]. Thus, these results suggest a role for SCFFBXL2 in regulating the fidelity of cellular division.
Keywords: :
Introduction
Cellular mitosis requires highly concerted actions of a network of regulatory proteins involved in cell cycle progression and proteolysis to ensure proper chromosomal segregation. For example, D-type cyclins are needed for G1/S cell cycle progression by interacting with cyclin-dependent kinases (Cdk 2, Cdk 4, Cdk 5 and Cdk 6).Citation1 Specifically, active cyclin D/Cdk 4 and cyclin D/Cdk 6 partially phosphorylate retinoblastoma tumor suppressor protein (Rb), thereby reducing its binding to E2F. This promotes cell progression to the S phase by allowing E2F-mediated activation of cyclin E gene transcription.Citation2,Citation3 Of the three major cyclin D family members (cyclin D1, cyclin D2 and cyclin D3), cyclin D1 has been well-studied, especially with regard to its role in G1/S-phase progression and in multi-organ tumorogenesis.Citation4–Citation6 Cyclin D1 protein is destabilized by a process involving its ubiquitination and proteosomal degradation. In particular, the F-box proteins within the SCF (Skp1-Cullin1-F-box) E3 ligase ubiquitin complex (FBXO4, FBXW8 and FBXO31) directly associate with and ubiquitinate cyclin D1.Citation7–Citation9 Unlike cyclin D1, less is known regarding the function and molecular control of cyclin D2 and cyclin D3. Cyclin D2 and cyclin D3 appear to display other roles in cellular division and intracellular signaling in addition to G1/S-phase transition.Citation10 Cyclin D3 appears unique in that it also partakes in G2/M-phase transition by interacting with CDK11p58 kinase during cell cycle progression.Citation11 CDK11p58 promotes centrosome maturation and bipolar spindle formation, and its interaction with cyclin D3 preserves CDK11p58 activity. Thus, the cyclin D3-CDK11p58 interaction appears to be vital during mitosis, and its abrogation could lead to G2 arrest.Citation11,Citation12
The SCF superfamily of ubiquitin E3 ligase proteins appear to be fundamentally involved in regulating cell cycle progression and mitosis.Citation13–Citation15 The SCF machinery consists of a catalytic core comprised of Skp1, Cullin1 and the E2 ubiquitin-conjugating (Ubc) enzyme.Citation16,Citation17 Within the SCF complex is a key receptor subunit, termed F-box protein, that binds to its substrates, usually through phosphospecific domain interactions.Citation18 Of the nearly 70 F-box proteins described, only ∼6 have defined roles in cellular processes.Citation19 Of note, the gene encoding the orphan F-box protein FBXL2 was strongly repressed in human lung adenocarcinoma.Citation20 After its initial description,Citation21 FBXL2 was shown to interact with the hepatitis C virus nonstructural protein 5A (NS5A), and this association was required for viral RNA replication.Citation22 NS5A and the phospholipid biosynthetic enzyme cytidylyltransferase are the only known targets of FBXL2, and its effects within the mitotic pathway have not been investigated.Citation23
Herein, we demonstrate that FBXL2 overexpression leads to cyclin D3 ubiquitination and its subsequent degradation in a SV40-tumorogenic cell line, resulting in mitotic arrest, an effect recapitulated by cyclin D3 knockdown. FBXL2 was localized to the centrosome, where it targets cyclin D3 for its ubiquitination. FBXL2-mediated ubiquitination and depletion of centrosomal cyclin D3 disrupted its association with CDK11p58 kinase and the multifunctional serine/threonine kinase, Aurora kinase, thus resulting in mitotic arrest.
Results
Overexpression of FBXL2 induces G2/M arrest and polyploidy by degrading cyclin D2 and cyclin D3.
We investigated FBXL2's effects on cell proliferation, first using human adenocarcinoma cells (A549) and then extended studies using a transformed murine lung epithelial (MLE) cell line. A plasmid encoding FBXL2 was first overexpressed in A549 cells, and after various times, cells were collected and stained with Annexin V, a marker of apoptosis, prior to FACS analysis (). To assess endogenous FBXL2, A549 cells were transfected with either scrambled RNA or siRNA targeting FBXL2, and cells were processed similiarly (). The results indicate that expression of FBXL2 reduces cell numbers, whereas knockdown of FBXL2 increased numbers of viable cells. Next, MLE cells were transfected with FBXL2, labled with BrdU and harvested for processing by two-color FACS after various times. The results indicate a significant increase in a cell poplulation within the G2/M phase, which accumulated over time. Interestingly, overexpression of FBXL2 tended to reduce the diploidal cell population and increase numbers of polyploidal cells in a time-dependent manner (). To investigate the mechanism by which FBXL2 induced G2/M arrest, we analyzed immunoreactive levels of cell cycle regulatory proteins after FBXL2 overexpression. Overexpression of the SCF subunit markedly reduced the levels of cyclin D2 and cyclin D3, two G1/S-phase regulators (). In contrast, levels of other G1 cyclins and the G2/M regulators cyclin A and cyclin B were unaffected. FBXL2 overexpression did not alter levels of other Cdks or negative control proteins (data not shown). To confirm that FBXL2 is an authentic E3 ligase and that its ability to decrease cyclin D3 mass may involve its ubiquitination, we conducted in vitro ubiquitination assays. Purified SCF complex, including FBXL2, was incubated with V5-cyclin D3 and the full complement of ubiquitination reaction components. Importantly, SCFFBXL2 with the full complement of E1 and E2 enzymes plus ubiquitin was sufficient to generate polyubiquitinated cyclin D3 in vitro ().
RNAi-mediated knockdown of cyclin D3 induces G2/M arrest and polyploidy.
MLE cells were transfected with either scrambled RNA or siRNA targeting cyclin D2 or cyclin D3. 72 h later, cells were harvested, and lysates were resolved on SDS-PAGE prior to immunoblotting (). In other experiments, cells were labled with BrdU and processed by two-color FACS. The results show that RNAi-mediated knockdown of cyclin D3, but not cyclin D2, resulted in a significant increase in a cell polulation within the G2/M phase (). Specifically, cells within the G2/M phase from cyclin D2 siRNA treatment showed a slight increase (34%) compared with control cells (31%), whereas the G2/M-phase population from cyclin D3 siRNA showed a substantial increase (47%) compared with control. Interestingly, the proportion of polyploidal cells also increased when cyclin D3 was depleted, mimicking effects of FBXL2 overexpression.
FBXL2 and cyclin D3 colocalize within the centrosome during mitosis.
As expressed, FBXL2 appears to impair mitosis; we investigated its subcellular localization throughout the cell cycle. We co-stained synchronized MLE cells with antibodies to FBXL2 and the centrosomal marker, γ-tubulin. FBXL2 antibody decorated cells in a punctate pattern but, interestingly, with specific localization within the centrosome throughout mitosis (). We next investigated the subcellular localization of cyclin D3. Endogenous cyclin D3 localizes primarily within the nucleus in the G1-S phase as overexpressed cyclin D3 (data not shown). We co-stained synchronized MLE cells with antibodies to cyclin D3 and γ-tubulin. Cyclin D3 antibody decorated cells in a punctate pattern during interphase and at the early prophase within the cytosol; progression into prometaphase was marked by an increase in signal intensity that colocalized with centrosomes. In general, cyclin D3 colocalized with γ-tubulin throughout the mitotic event ().
Overexpression of FBXL2 depletes cyclin D3 on the centrosome.
As FBXL2 and cyclin D3 localized on the centrosome, we next investigated the effect of FBXL2 overexpression on cyclin D3. MLE cells were first transfected with either empty plasmid or a plasmid encoding FBXL2. Cells were then immunostained with FBXL2 and γ-tubulin. As shown in , overexpression of FBXL2 increased its expression in a region that colocalized with the centrosome. In other studies, cells were then immunostained with cyclin D3 and γ-tubulin. Overexpression of FBXL2 depleted both cytosolic cyclin D3 and cyclin D3 within the centrosome ().
Overexpression of FBXL2 causes centrosomal and mitotic aberrations.
We next examined the integrity of the centrosome and mitotic spindles after expression of FBXL2. Abnormal cells with several representative mitotic abnormalities were detected after FBXL2 expression (). We observed the circular prophase and prometaphase-like configurations (, upper part) together with anaphase figures, in which chromosomes appear to be arranged in circles around both poles (, lower right part). One classic phenotype of the circular chromosome configuration is that circular figures are arranged on monopolar spindles around large centrosomes. We also observed polyploidy in cells with metaphase, where multiple centrosomes were identified after FBXL2 expression (, lower left part). Events in which numbers of mitotic cells with both monopolar or multipolar spindles with lagging chromosomes significantly increased after FBXL2 expression were quantified ().
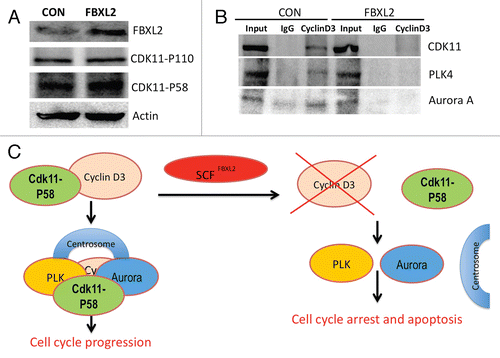
Overexpression of FBXL2 impairs assembly of the cyclin D3/CDK11 complex.
As stated above, the cyclin D3-CDK11p58 interaction appears to be critical to ensure mitotic progression, and its abrogation leads to G2 arrest. CDK11 also recruits polo-like kinase 4 (PLK4) and Aurora A to the centrosome, which also regulates mitotic events and chromosomal stability.Citation24 Thus, to determine the mechanism of mitotic aberrations upon FBXL2 overexpression, the interaction between cyclin D3 and CDK11 was assessed. Cells were transfected with either empty plasmid or FBXL2, cells were harvested, and lysates were resolved on SDS-PAGE prior to CDK11 immunoblots. Overexpression of FBXL2 did not alter total immunoreactive levels of the p110 and p58 subunits of CDK11 (). Next, cellular transfectants were immunoprecipitated with IgG or cyclin D3 antibodies, followed by CDK11, PLK4 and Aurora A immunoblotting (). The results indicate that overexpression of FBXL2 disrupts the interaction between cyclin D3 and CDK11 and association with PLK4 and Aurora A.
Discussion
The results from this study provide the first evidence that high levels of F-box protein FBXL2 appear to impair G2/M transition and, thus, might serve as a key growth inhibitory signal. F-box protein degradation of cyclin D2 and cyclin D3 resulting in G2/M arrest was unexpected (), as these cyclins regulate G1/S transition, and knockdown of cyclin D3 has been shown to induce G1 arrest.Citation25 However, both cyclin D2 and cyclin D3 may be necessary for G2/M-phase progression.Citation11,Citation26 Moreover, cyclin D3 is a multifunctional protein that directly interacts with and confers activity for CDK11p58,Citation11,Citation12 a key cyclin-dependent kinase that controls centrosome maturation and bipolar spindle formation.Citation24 The results shown here suggest that one mechanism whereby FBXL2 might induce mitotic arrest is by depleting cyclin D3, leading to absence of its interactions with critical effectors that assemble within the centrosomal-mitotic apparatus ().
During mitosis, there are several mediators involved in spindle assembly checkpoint, ensuring the correct distribution of sister chromatids in anaphase prior to completion of cytokinesis by abscission.Citation27,Citation28 Our data show that both cyclin D3 and FBXL2 are localized within the centrosome during mitosis ( and ), and that expression of FBXL2 reduces cyclin D3 expression within this organelle (). As with FBXL2 in cells, knockdown of CDK11p58 results in G2 arrest and apoptosis; significant CDK11 depletion results in misaligned and lagging chromosomes, permanent mitotic arrest and cell death.Citation29 Hence, SCFFBXL2-mediated ubiquitination and degradation of cyclin D3 would potentially impair its association with CDK11p58 and reduce its activity. In support of this, overexpression of FBXL2 does not decrease total cellular CDK11 protein levels (), but reduces assembly of the cyclin D3-CDK11p58 complex (). One additional function of CDK11 is to recruit polo-like kinase 4 (PLK4) and Aurora A to the centrosome, which also regulates mitotic events and chromosomal stability.Citation24 Knockout of PLK4 results in increased centrosome number and aneuploidy,Citation30 and expression of a defective mutant Aurora protein prevents centrosome separation, leading to formation of mono-polar spindles.Citation31 These features were also observed after FBXL2 expression in cells (). Collectively, these results suggest that one explanation for G2/M-phase delay and mitotic abnormalities might involve SCFFBXL2 inactivation of CDK11p58 by depletion of cyclin D3. This mechanism would dislocate PLK4 and Aurora A, causing mitotic arrest ().
Compared with cyclin D2, cyclin D3 is more dominant in MLE cells, as RNA silencing of cyclin D3 showed significant increases (∼50%) in the G2 phase, whereas knockdown of cyclin D2 showed limited effects. Similiar to overexpressed FBXL2, knockdown of cyclin D3 and, to a lesser extent, cyclin D2, resulted in G2/M arrest and appearance of supernumerary centrosomes and tetraploidy (). Because both cyclins are highly conserved and exert some redundant functions, a more modest phenotype observed with cyclin D2 knockdown might be because of compensation by cyclin D3. This would occur especially if lower levels of cyclin D2 are present in MLE cells compared with cyclin D3 as seen with A549 cells and CHO cells (data not shown). The data presented here suggest a new mechanism whereby F-box protein FBXL2 regulates mitotic events through control of cyclin D3 abundance. Interestingly, in separate studies, the chemotherapeutic agent vinorelbine increases apoptosis of human lung carcinoma cells by inducing FBXL2 expression and triggering cyclin D3 degradation (unpublished observations). Thus, FBXL2 targeting cyclin D3 might be a new mechanism by which cells modulate proliferation. As stated above, cyclin D3-dependent CDK11p58 activity is essential for mitosis, but excessive CDK11p58 levels also repress cellular proliferation and induce apoptosis.Citation12 Hence, cyclin D3 targeting by the SCFFBXL2 complex during the transition to mitosis might be an exquisite mechanism to balance CDK11p58 levels, thereby regulating cell proliferation.
Methods and Materials
Materials.
The sources of the transformed murine lung epithelial (MLE) cell line, A549 cell line, were described previously in reference Citation32 and Citation33. Cyclin and Cdk sampler kits and γ-tubulin, Aurora A and PLK antibodies were purchased from Cell Signaling. Mouse monoclonal cyclin D2, cyclin D3 and CDK11 antibodies and were from Abcam. Lipofectamine 2000, mouse monoclonal V5 antibody and DAPI nuclear staining kits were from Invitrogen. The F-box protein FBXL2 cDNA was purchased from OpenBiosystems. Nucleofector transfection kits were from Amaxa. Immobilized protein A/G beads were from Pierce. Cell viability based on Annexin V staining was assayed using an Annexin V kit from Roche. Goat polyclonal FBXL2 antibody, scrambled RNA and siRNAs were from Santa Cruz Biotechnology. Rabbit polyclonal FBXL2 antibody was custom made from Rockland Immunochemicals Inc. All DNA sequencing was performed by the University of Pittsburgh DNA Core Facility.
Cell culture.
MLE cells were cultured in Dulbecco's modified Eagle medium-F12 (Gibco) supplemented with 10% fetal bovine serum (DMEM-F12 10%). A549 cells were cultured in MEM (Gibco) supplemented with 10% fetal bovine serum (MEM-10). Cells in some studies were synchronized using serum starvation (DMEM-F12) for 48 h or treatment with nocodazole or aphidicolin. Cell lysates were prepared by brief sonication in 150 mM NaCl, 50 mM Tris, 1.0 mM EDTA, 2 mM dithiothreitol, 0.025% sodium azide and 1 mM phenylmethylsulfonyl fluoride (Buffer A) at 4°C.
In vitro ubiquitin conjugation assay.
The ubiquitination of V5-cyclin D3 was performed in a volume of 25 µl containing 50 mM Tris pH 7.6, 5 mM MgCl2, 0.6 mM DTT, 2 mM ATP, 1.5 ng/µl E1, 10 ng/µl Ubc5, 10 ng/µl Ubc7, 1 µg/µl ubiquitin (Calbiochem), 1 µM ubiquitin aldehyde, 4–16 µl of purified Cullin1, Skp1, Rbx1 and FBXL2. Reaction products were processed for V5 immunoblotting.
Expression of recombinant protein and RNAi.
All plasmids were delivered into cells using nucleofection or lipofectamine 2000.Citation34,Citation35 Cellular expression of green fluorescent-tagged plasmids using this device was achieved at > 90% efficiency. For siRNA studies, 1 × 106 cells were transfected using lipofectamine 2000 with 10 µg of RNA and harvested after an additional 48 h.
Co-immunoprecipitation and binding assays.
250 µg of total protein from cell lysates was precleared with 20 µl of protein A/G beads for 1 h at 4°C. Five µg of primary antibody was added for 18 h incubation at 4°C. 40 µl of protein A/G beads were added for an additional 6 h of incubation. Beads were slowly centrifuged and washed five times using 50 mM HEPES, 150 mM NaCl, 0.5 mM EGTA, 50 mM NaF, 10 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 20 µM leupeptin and 1% (v/v) Triton X-100 (RIPA) buffer, as described in reference Citation36. The beads were heated at 100°C for 5 min with 80 µl of protein sample buffer prior to SDS-PAGE and immunoblotting.
Immunostaining.
Cells (2 × 105) were plated at 70% confluence on 35 mm MatTek glass-bottom culture dishes. Immunofluorescent cell imaging was performed on a Nikon A1 confocal microscope using 405 nm, 458 nm, 488 nm, 514 nm or 647 nm wavelengths. All experiments were done with a 60x oil differential interference contrast objective lens. Cells were washed with PBS and fixed with 4% paraformaldehyde for 20 min, then exposed to 15% BSA, 1:500 primary antibodies and 1:1,000 Alexa 488 or Alexa 647 labeled goat anti-mouse or rabbit secondary antibody sequentially for immunostaining.
Cell cycle and apoptosis analysis.
Transfected cells were incubated with BrdU (20 µM) for 40 min, fixed and stained following manufacturer's protocols (BD Biosciences). FACS samples were analyzed with the AccuriC6 system. DNA content was analyzed using FCS3 express software (De Novo Software). Cells were counted, and the percentage of cells with 2N, 4N and 8N DNA content was expressed as a percentage of total cells. Cells were also stained with annexin V for 15 min following the manufacturer's protocol (Roche). Viable cells were counted and quantified.
Statistical analysis.
Statistical comparisons were performed with the Prism program, version 4.03 (GraphPad Software, Inc.) using an ANOVA 1 or an unpaired two-tailed t-test with p < 0.05 indicative of significance.
Disclosure of potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures and Tables
Figure 1 Overexpression of FBXL2 induces G2/M arrest by selectively degrading cyclin D2 and cyclin D3. (A) FACS analysis of A549 cells transfected with either empty vector or FBXL2 plasmid; viable cells were quantified and graphed (n = 3). (B) FACS analysis of A549 cells transfected with either scrambled RNA or FBXL2 siRNA; viable cells were quantified and graphed (n = 3). (C) Cell cycle analysis. MLE cells were transfected with FBXL2 and cells analyzed over time. At each time point, cells were analyzed by BrdU uptake and 7-AAD staining; 2n, 4n and 8n DNA contents were then quantitated and graphed after expression of FBXL2. (D) Immunoblotting showing levels of cyclins and negative control protein actin after control (CON) plasmid or FBXL2 expression (n = 2 experiments). (E) In vitro ubiquitination assay. Purified SCF complex, including FBXL2, were incubated with V5-cyclin D3 and the full complement of ubiquitination reaction components. Reactions products were processed for SDS-PAGE and immunoblotting.
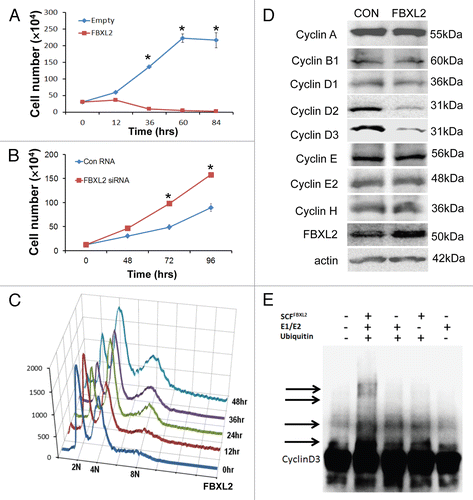
Figure 2 RNAi-mediated knockdown of cyclin D3 induces G2/M arrest and polyploidy. (A) MLE cells were transfected with either scrambled RNA or cyclin D2 siRNA or cyclin D3 siRNA. 72 h later, cells were harvested; lysates were resolved on SDS-PAGE prior to cyclin D2 or cyclin D3 immunoblotting. (B) Transfected cells were analyzed by BrdU uptake and 7-AAD staining; 2n, 4n and 8n DNA contents were quantitated and graphed (n = 2 experiments).
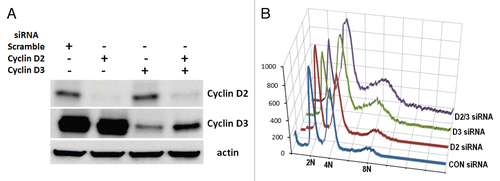
Figure 3 FBXL2 is localized on the centrosome during mitosis. MLE cells (2 × 105) were plated for 48 h then washed with PBS and fixed with 4% paraformaldehyde for 20 min. Cells were co-immunostained for FBXL2 and γ-tubulin. Nuclei were counterstained using DAPI. Green, γ-tubulin; red, FBXL2; blue, DAPI. The far right column shows a graphic distribution of protein colocalization indicated by the white arrow. White scale bar indicates 10 µm.
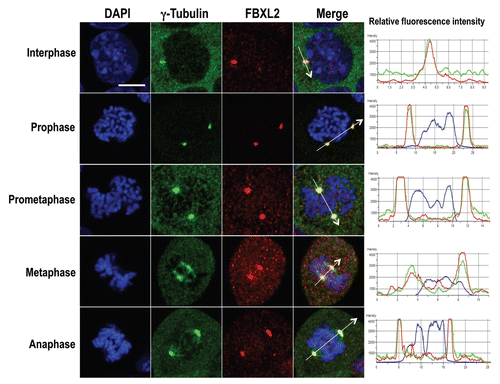
Figure 4 Cyclin D3 is localized on the centrosome during mitosis. MLE cells (2 × 105) were plated for 48 h, cells were then washed with PBS and fixed with 4% paraformaldehyde for 20 min. Cells were co-immunostained for cyclin D3 and γ-tubulin. Nucleus was counterstained using DAPI. Green, cyclin D3; red, γ-tubulin; blue, DAPI. The far right column shows a graphic distribution of protein colocalization indicated by the white arrow. White scale bar indicates 10 µm.
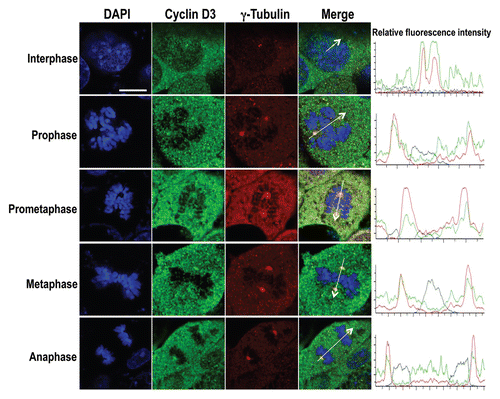
Figure 5 Overexpression of FBXL2 depletes cyclin D3 on the centrosome. MLE cells (2 × 105) were plated for 24 h, then transfected with FBXL2 for an additional 24 h. Cells were then washed with PBS and fixed with 4% paraformaldehyde for 20 min. Cells were co-immunostained for either γ-tubulin or FBXL2 (A), or cyclin D3 and γ-tubulin (B). Cells were counterstained with DAPI to visualize the nucleus. White arrows indicate centrosomes. White scale bar indicates 2 µm.
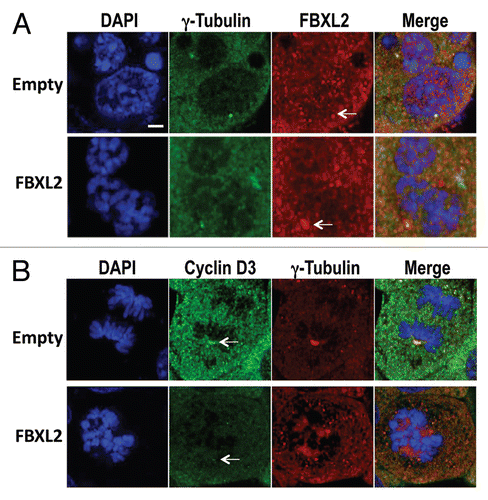
Figure 6 Overexpression of FBXL2 causes centrosomal and mitotic aberrations. (A) MLE cells (2 × 105) were plated for 24 h, then transfected with FBXL2 for additional 24 h. Cells were then immunostained for γ-tubulin and counterstained with DAPI to visualize the nucleus. White arrows represent cells in mitotic arrest where condensed chromosomes are arranged on circular monopolar spindles. Specific chromosomal anomalies are presented in (B). (C) 100 cells from three individual experiments were counted from experiments in (A) for abnormal centrosomal phenotypes and are presented graphically. *p < 0.05 vs. empty.
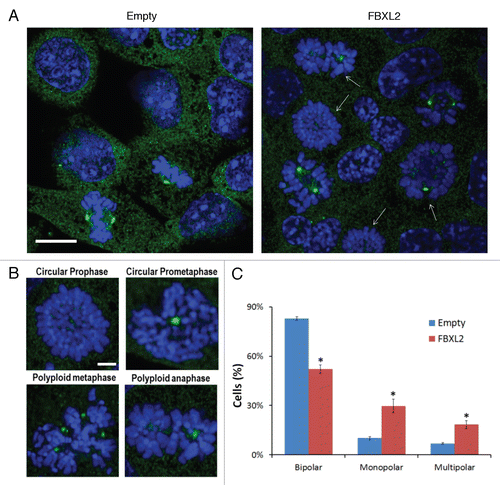
Figure 7 Overexpression of FBXL2 dissociates the cyclin D3/CDK11 complex. (A) MLE cells were transfected with either empty or FBXL2 plasmid. Twenty-four h later, cells were collected, lysed and immunoblotted with antibodies to the indicated proteins. (B) Cyclin D3 was immunoprecipitated from lysates in (A), followed by immunoblotting to the indicated proteins.
Acknowledgments
We thank A.F. Stewart for critical review of the manuscript and helpful suggestions. This material is based upon work supported, in part, by the US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. This work was supported by a Merit Review Award from the US Department of Veterans Affairs and National Institutes of Health R01 grants HL096376, HL097376 and HL098174 (to R.K.M.). The contents presented do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- Lew DJ, Dulic V, Reed SI. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell 1991; 66:1197 - 1206; PMID: 1833066; http://dx.doi.org/10.1016/0092-8674(91)90042-W
- Ohtani K, DeGregori J, Nevins JR. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci USA 1995; 92:12146 - 12150; PMID: 8618861; http://dx.doi.org/10.1073/pnas.92.26.12146
- Peeper DS, Upton TM, Ladha MH, Neuman E, Zalvide J, Bernards R, et al. Ras signalling linked to the cell cycle machinery by the retinoblastoma protein. Nature 1997; 386:177 - 181; PMID: 9062190; http://dx.doi.org/10.1038/386177a0
- Gautschi O, Ratschiller D, Gugger M, Betticher DC, Heighway J. Cyclin D1 in non-small cell lung cancer: a key driver of malignant transformation. Lung Cancer 2007; 55:1 - 14; PMID: 17070615; http://dx.doi.org/10.1016/j.lungcan.2006.09.024
- Zhang Q, Tian L, Mansouri A, Korapati AL, Johnson TJ, Claret FX. Inducible expression of a degradation-resistant form of p27Kip1 causes growth arrest and apoptosis in breast cancer cells. FEBS Lett 2005; 579:3932 - 3940; PMID: 15996662; http://dx.doi.org/10.1016/j.febslet.2005.06.012
- Zi X, Agarwal R. Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: implications for prostate cancer intervention. Proc Natl Acad Sci USA 1999; 96:7490 - 7495; PMID: 10377442; http://dx.doi.org/10.1073/pnas.96.13.7490
- Li W, Sanki A, Karim RZ, Thompson JF, Soon Lee C, Zhuang L, et al. The role of cell cycle regulatory proteins in the pathogenesis of melanoma. Pathology 2006; 38:287 - 301; PMID: 16916716; http://dx.doi.org/10.1080/00313020600817951
- Okabe H, Lee SH, Phuchareon J, Albertson DG, McCormick F, Tetsu O. A critical role for FBXW8 and MAPK in cyclin D1 degradation and cancer cell proliferation. PLoS ONE 2006; 1:128; PMID: 17205132; http://dx.doi.org/10.1371/journal.pone.0000128
- Santra MK, Wajapeyee N, Green MR. F-box protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest after DNA damage. Nature 2009; 459:722 - 725; PMID: 19412162; http://dx.doi.org/10.1038/nature08011
- Tanguay DA, Colarusso TP, Doughty C, Pavlovic-Ewers S, Rothstein TL, Chiles TC. Cutting edge: differential signaling requirements for activation of assembled cyclin D3-cdk4 complexes in B-1 and B-2 lymphocyte subsets. J Immunol 2001; 166:4273 - 4277; PMID: 11254678
- Zhang S, Cai M, Xu S, Chen S, Chen X, Chen C, et al. Interaction of p58(PITSLRE), a G2/M-specific protein kinase, with cyclin D3. J Biol Chem 2002; 277:35314 - 35322; PMID: 12082095; http://dx.doi.org/10.1074/jbc.M202179200
- Duan Y, He X, Yang H, Ji Y, Tao T, Chen J, et al. Cyclin D3/CDK11(p58) complex involved in Schwann cells proliferation repression caused by lipopolysaccharide. Inflammation 2010; 33:189 - 199; PMID: 20066559; http://dx.doi.org/10.1007/s10753-009-9173-8
- Pagano M. Control of DNA synthesis and mitosis by the Skp2-p27-Cdk1/2 axis. Mol Cell 2004; 14:414 - 416; PMID: 15149588; http://dx.doi.org/10.1016/S1097-2765(04)00268-0
- Tyers M, Willems AR. One ring to rule a super-family of E3 ubiquitin ligases. Science 1999; 284:601 - 603; PMID: 10328744; http://dx.doi.org/10.1126/science.284.5414.601
- Moshe Y, Boulaire J, Pagano M, Hershko A. Role of polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc Natl Acad Sci USA 2004; 101:7937 - 7942; PMID: 15148369; http://dx.doi.org/10.1073/pnas.0402442101
- Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature 2002; 416:703 - 709; PMID: 11961546; http://dx.doi.org/10.1038/416703a
- Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol 2004; 5:739 - 751; PMID: 15340381; http://dx.doi.org/10.1038/nrm1471
- Cenciarelli C, Chiaur DS, Guardavaccaro D, Parks W, Vidal M, Pagano M. Identification of a family of human F-box proteins. Curr Biol 1999; 9:1177 - 1179; PMID: 10531035; http://dx.doi.org/10.1016/S0960-9822(00)80020-2
- Skaar JR, Pagan JK, Pagano M. SnapShot: F-box proteins I. Cell 2009; 137:1160 - 1161; PMID: 19524517; http://dx.doi.org/10.1016/j.cell.2009.05.039
- Malard V, Berenguer F, Prat O, Ruat S, Steinmetz G, Quemeneur E. Global gene expression profiling in human lung cells exposed to cobalt. BMC Genomics 2007; 8:147; PMID: 17553155; http://dx.doi.org/10.1186/1471-2164-8-147
- Ilyin GP, Rialland M, Glaise D, Guguen-Guillouzo C. Identification of a novel Skp2-like mammalian protein containing F-box and leucine-rich repeats. FEBS Lett 1999; 459:75 - 79; PMID: 10508920; http://dx.doi.org/10.1016/S0014-5793(99)01211-9
- Wang C, Gale M Jr, Keller BC, Huang H, Brown MS, Goldstein JL, et al. Identification of FBL2 as a geranylgeranylated cellular protein required for hepatitis C virus RNA replication. Mol Cell 2005; 18:425 - 434; PMID: 15893726; http://dx.doi.org/10.1016/j.molcel.2005.04.004
- Chen BB, Coon TA, Glasser JR, Mallampalli RK. Calmodulin antagonizes a calcium-activated SCF ubiquitin E3 ligase subunit, FBXL2, to regulate surfactant homeostasis. Mol Cell Biol 2011; 31:1905 - 1920; PMID: 21343341
- Petretti C, Savoian M, Montembault E, Glover DM, Prigent C, Giet R. The PITSLRE/CDK11p58 protein kinase promotes centrosome maturation and bipolar spindle formation. EMBO Rep 2006; 7:418 - 424; PMID: 16462731
- Sicinska E, Aifantis I, LeCam L, Swat W, Borowski C, Yu Q, et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 2003; 4:451 - 461; PMID: 14706337; http://dx.doi.org/10.1016/S1535-6108(03)00301-5
- Fang MZ, Lee MH, Lee YS, Kim YC, Lee BM, Cho MH. Low expression of cyclin D2 in G2/M-arrested and transformed proliferating Balb/3T3 cells. J Vet Med Sci 2002; 64:201 - 205; PMID: 11999438; http://dx.doi.org/10.1292/jvms.64.201
- Ho CC, Hau PM, Marxer M, Poon RY. The requirement of p53 for maintaining chromosomal stability during tetraploidization. Oncotarget 2010; 1:583 - 595; PMID: 21317454
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The active form of the metabolic sensor: AMP-activated protein kinase (AMPK) directly binds the mitotic apparatus and travels from centrosomes to the spindle midzone during mitosis and cytokinesis. Cell Cycle 2009; 8:2385 - 2398; PMID: 19556893; http://dx.doi.org/10.4161/cc.8.15.9082
- Hu D, Valentine M, Kidd VJ, Lahti JM. CDK11(p58) is required for the maintenance of sister chromatid cohesion. J Cell Sci 2007; 120:2424 - 2434; PMID: 17606997; http://dx.doi.org/10.1242/jcs.007963
- Rosario CO, Ko MA, Haffani YZ, Gladdy RA, Paderova J, Pollett A, et al. Plk4 is required for cytokinesis and maintenance of chromosomal stability. Proc Natl Acad Sci USA 2010; 107:6888 - 6893; PMID: 20348415; http://dx.doi.org/10.1073/pnas.0910941107
- Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell 1995; 81:95 - 105; PMID: 7720077; http://dx.doi.org/10.1016/0092-8674(95)90374-7
- Ray NB, Durairaj L, Chen BB, McVerry BJ, Ryan AJ, Donahoe M, et al. Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nat Med 2010; 16:1120 - 1127; PMID: 20852622; http://dx.doi.org/10.1038/nm.2213
- Chen BB, Mallampalli RK. Calmodulin binds and stabilizes the regulatory enzyme, CTP: phosphocholine cytidylyltransferase. J Biol Chem 2007; 282:33494 - 33506; PMID: 17804406; http://dx.doi.org/10.1074/jbc.M706472200
- Agassandian M, Chen BB, Schuster CC, Houtman JC, Mallampalli RK. 14-3-3zeta escorts CCTalpha for calcium-activated nuclear import in lung epithelia. FASEB J 2010; 24:1271 - 1283; PMID: 20007511; http://dx.doi.org/10.1096/fj.09-136044
- Chen BB, Mallampalli RK. Masking of a nuclear signal motif by monoubiquitination leads to mislocalization and degradation of the regulatory enzyme cytidylyltransferase. Mol Cell Biol 2009; 29:3062 - 3075; PMID: 19332566; http://dx.doi.org/10.1128/MCB.01824-08
- Mallampalli RK, Ryan AJ, Salome RG, Jackowski S. Tumor necrosis factoralpha inhibits expression of CTP:phosphocholine cytidylyltransferase. J Biol Chem 2000; 275:9699 - 9708; PMID: 10734122; http://dx.doi.org/10.1074/jbc.275.13.9699