Abstract
Comment on: Lee MH, et al. Mol Cell 2011; 43:180-91.
SUMOylation of protein substrates and the transcription factor NFκB family are both linked to a myriad of physiological and pathological processes.Citation1–Citation3 However, the known connections between SUMO and NFκB signaling are currently limited. Examples include SUMO-1 modification of IκBα, the main inhibitor of canonical NFκB dimers, which prevents signal-induced ubiquitination and degradation of IκBα and thus limits NFκB activation.Citation4 SUMO-2/3 modification of the same lysine residue of IκBα, in contrast, seems to impart dissociation of NFκB from IκBα to induce NFκB activation in response to hypoxia.Citation5 Similarly, basal SUMO-1 modification of p100 is thought to serve as an essential permissive mechanism to allow for non-canonical NFκB activation.Citation6 In these cases, specific SUMO ligase or SUMO-specific protease (SENP) that mediates direct SUMOyation or deSUMOylation of respective substrate remains unclear. Another example is SUMO-1 modification of NEMO (NFκB Essential MOdulator) that mediates NFκB activation in response to genotoxic stress.Citation7 In this case, the critical ligase is defined as PIASy (Protein Inhibitor of Activated Stat y, also known as PIASγ/PIAS4). Different nuclear proteins may also participate in this modification process, including PIDD (p53-induced protein with death domain), RIP1 (receptor-interacting protein 1) and PARP1 [poly-(ADP ribose) polymerase 1].Citation7 In a recent study,Citation8 we reported the identification of SENP2 (Sentin/SUMO-specific protease 2), among the six known SUMO pro-teases, as the major SUMO protease for NEMO. This study also provided a new concept that SUMO and NFκB ties are not simply one way but can be bidirectional, SUMO regulating NFκB signaling on one hand, and NFκB modulating SUMOylation via induction of SUMO proteases on the other, thereby implicating new roles for the NFκB system.
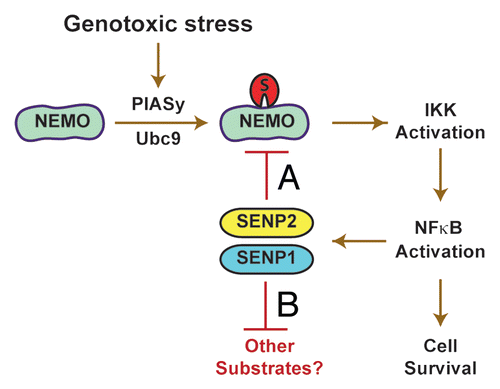
One fundamental feature of the NFκB signaling system is the presence of negative feedback regulation.Citation9 It appears that this control mechanism has evolved to tightly regulate its own activity, because uncontrolled NFκB activity (both excessive and deficient) contributes to pathological disease states.Citation1 We revealed that the gene encoding SENP2 (and SENP1 but not PIASy) is a novel, direct transcriptional target of NFκB, which is induced after DNA damage signals to attenuate upstream IKK activation via deSU-MOylation of NEMO (). This response is signal-specific and is coupled to DNA damage activation of the ATM (Ataxia telangiectasia mutated) protein kinase, ATM-dependent histone methylation of the SENP2 locus, and NFκB recruitment to regulatory κB binding sites located there. SENP2-deficient cells display both heightened and prolonged (biphasic) NFκB response accompanied by correspondingly increased NEMO SUMOylation and IKK activation. These cells also display increased cell survival in response to genotoxic stress in a manner dependent on NFκB activation. Thus, SENP2 induction is engaged to feedback attenuate NFκB-mediated cell survival response in the face of DNA damage stress.
Given that post-translational modification of many protein substrates by SUMOylation impacts an array of processes and deSUMOylation needs to be mediated by a very small number of SUMO proteases, we speculate that NFκB induction of SENP2 (and SENP1) modulates other processes in addition to NEMO deSUMOylation (). For example, activities of many transcriptional regulators are regulated by SUMOylation, including p53, Fos, Jun and HDAC1, among others.Citation2,Citation3 SENP2 is implicated in cell cycle regulation mediated by the p53-Mdm2 pathway, while SENP1 appears to regulate development of prostate cancer involving modulation of androgen receptor activity.Citation2 Finally, the DNA damage responsive factors, BRCA1 and 53BP1, are SUMO modified to regulate DNA repair.Citation10,Citation11 While different proteins can be modified by SUMO-1 or −2/3 (or both), SENP2 and SENP1 can deconjugate SUMO-1 or −2/3, depending on the substrates.Citation2,Citation3 Thus, definition of SENP2/1 as direct NFκB transcriptional targets provides an initial mechanism for NFκB regulation of SUMO pathways. Elucidation of additional mechanisms for bidirectional regulation between SUMO and NFκB pathways will increase our understanding of their roles in normal and disease processes.
Figures and Tables
Figure 1 Feedback regulation of NFκB signaling induced by genotoxic agents. (A) NFκB activation by genotoxic stress involves SUMoylation of NEMO via PIASy (E3) and Ubc9 (E2), which leads to IKK activation. NFκB then activates the synthesis of SENP1 and SENP2 genes. SENP2 serves as the primary SUMO protease for NEMO, which leads to feedback attenuation of IKK and NFκB activation. (B) NFκB-dependent induction of SENP1 and SENP2 modulates SUMOylation status of other substrates.
Comment on: Lee MH, et al. Mol Cell 2011; 43:180 - 191; PMID: 21777808; http://dx.doi.org/10.1016/j.molcel.2011.06.017
References
- Perkins ND. Nat Rev Mol Cell Biol 2007; 8:49 - 62; PMID: 17183360; http://dx.doi.org/10.1038/nrm2083
- Yeh ET. J Biol Chem 2009; 284:8223 - 8227; PMID: 19008217; http://dx.doi.org/10.1074/jbc.R800050200
- Gareau JR, et al. Nat Rev Mol Cell Biol 2010; 11:861 - 871; PMID: 21102611; http://dx.doi.org/10.1038/nrm3011
- Desterro JM, et al. Mol Cell 1998; 2:233 - 239; PMID: 9734360; http://dx.doi.org/10.1016/S10972765(00)80133-1
- Culver C, et al. Mol Cell Biol 2010; 30:4901 - 4921; PMID: 20696840; http://dx.doi.org/10.1128/MCB.00409-10
- Vatsyayan J, et al. EMBO Rep 2008; 9:885 - 890; PMID: 18617892; http://dx.doi.org/10.1038/embor.2008.122
- Miyamoto S. Cell Res 2011; 21:116 - 130; PMID: 21187855; http://dx.doi.org/10.1038/cr.2010.179
- Lee MH, et al. Mol Cell 2011; 43:180 - 191; PMID: 21777808; http://dx.doi.org/10.1016/j.molcel.2011.06.017
- Renner F, et al. Trends Biochem Sci 2009; 34:128 - 135; PMID: 19233657; http://dx.doi.org/10.1016/j.tibs.2008.12.003
- Morris JR, et al. Nature 2009; 462:886 - 890; PMID: 20016594; http://dx.doi.org/10.1038/nature08593
- Galanty Y, et al. Nature 2009; 462:935 - 939; PMID: 20016603; http://dx.doi.org/10.1038/nature08657