Abstract
Autophagy is a cellular survival pathway that recycles intracellular components to compensate for nutrient depletion and ensures the appropriate degradation of organelles. Mitochondrial number and health are regulated by mitophagy, a process by which excessive or damaged mitochondria are subjected to autophagic degradation. Autophagy is thus a key determinant for mitochondrial health and proper cell function. Mitophagic malfunction has been recently proposed to contribute to progressive neuronal loss in Parkinson's disease. In addition to autophagy's significance in mitochondrial integrity, several lines of evidence suggest that mitochondria can also substantially influence the autophagic process. The mitochondria's ability to influence and be influenced by autophagy places both elements (mitochondria and autophagy) in a unique position where defects in one or the other system could increase the risk to various metabolic and autophagic related diseases.
Introduction
Autophagy is a cellular degradation system that is highly conserved among different eukaryotic species. Since the discovery of this pathway over 40 years ago, the identification of autophagy-regulating proteins (ATG) has tremendously increased our understanding of how autophagy functions.Citation1,Citation2 The orchestrated activation of the pro-autophagic Beclin1/PI3K complex and recruitment of ATG proteins induces the formation of autophagosomes. These double membranous vesicles sequester and degrade cytoplasmic materials. Among the autophagosomal substrates are cytosolic proteins, ribosomes and organelles (such as mitochondria and the ER) as well as bacteria and viruses.Citation3 The enormous variety of substrates explains the close link between autophagy defects and diverse human diseases, including cancer and neurodegenerative disorders. Seminal studies by Youle and colleagues identified the Parkinson disease-associated proteins Pink1 and Parkin as mediators of the selective degradation of dysfunctional mitochondria by autophagy (termed mitophagy).Citation4 Disease-associated Parkin mutants caused loss of mitophagy upon mitochondrial damage,Citation5,Citation6 suggesting that the accumulation of damaged mitochondria could contribute to the mechanism of Parkinson disease.
The selective degradation of mitochondria by autophagy controls mitochondrial number and health. Besides being a substrate for autophagy, accumulating evidence indicates that mitochondria themselves can influence the autophagic process in several ways. To date, mitochondria have been linked to virtually every step of autophagy, including autophagosomal biogenesis and autophagic flux.Citation7–Citation9 In this short review, we summarize the possible means by which mitochondria and autophagy crosstalk, with an emphasis on the mitochondrial control of autophagy in mammalian cells. The strong interconnection between the mitochondrial and autophagic systems could potentiate the contribution of both systems for neurodegenerative, inflammatory and cancer-related diseases.
Mitochondria: A Dynamic Organelle
Mitochondria are highly dynamic organelles, with morphologies ranging from small roundish elements to larger interconnected networks. Mitochondrial architecture is not random, rather the opposing processes of fission and fusion specifically determine mitochondrial shape. On the molecular level, mitochondrial morphology is controlled through a family of dynamin-related proteins. Mitofusin 1 and 2 (Mfn1, Mfn2) and optic atrophic protein 1 (Opa1) fuse mitochondria,Citation10 while fission is mainly regulated by the Dynamin-related protein1 (Drp1) and mitochondrial fission factor (Mff).Citation11,Citation12
The dynamic nature of mitochondria allows the adjustment of mitochondrial morphologies to specific cellular processes. For example, before cells enter the energy-costly DNA replication phase (S phase) mitochondria become hyperfused and increase their ATP production.Citation13 Different cellular pathways can regulate the activity of mitochondria-shaping proteins and adapt mitochondrial architecture to the cell's state.Citation14 Our best understanding of this regulation relates to Drp1 activity. Multiple posttranslational modifications, including phosphorylation, sumoylation and ubiquitination events, regulate Drp1 activity and thus mitochondrial division.Citation11,Citation14 Mitochondrial shape is also determined through the activity of fusion proteins. The E3 ligase March5 has been identified to regulate fusion through targeting of Mfn1 and/or Mfn2.Citation15,Citation16 In addition, fusion is determined through mitochondrial membrane potential, as the activity of the inner membrane fusion protein Opa1 is voltage-dependent.Citation17–Citation20
The specific control of mitochondrial morphology has a significant impact on mitochondrial function and homeostasis. Mitochondrial fusion was suggested as a route for the rapid exchange of metabolites, mitochondrial DNA (mtDNA) and membrane components,Citation21–Citation27 while fission is thought to facilitate the segregation of mtDNA and isolation of mitochondria from the network to allow their degradation.Citation28–Citation32 Through this, mitochondrial fission and fusion influences nearly all aspects of mitochondrial function, including respiration, calcium buffering and apoptosis.Citation33–Citation37
The dynamic nature of mitochondria is also essential for mitochondrial quality control. Healthy mitochondria go through continuous fission and fusion cycles, which are, in general, timely coupled. In this process, after fusion takes place, it is rapidly followed by a mitochondrial fission event. Mitochondria then spend the vast amount of time in a post-fission state, which they only leave by re-fusing into the mitochondrial network. As mitochondrial fusion is dependent on membrane potential (Δψ m), mitochondrial depolarization will retain mitochondria in a post-fissioned state.Citation17–Citation20,Citation32 The continuous failure of damaged mitochondria to fuse back into the mitochondrial network eventually leads to mitochondrial elimination through autophagy.Citation32 Mitochondrial dynamics thereby provides the cell with a powerful mechanism to regulate the number and overall health of mitochondria.
Mitochondria as an Autophagy Substrate
One link between autophagy and mitochondria is the selective elimination of excess or damaged mitochondria, a process called mitophagy. Mitochondria are degraded under a variety of different conditions, including basal mitochondrial quality control,Citation32 mitochondrial dysfunctionCitation4,Citation32 and during developmental processes, such as during the maturation of immature red blood cells.Citation38–Citation40 To date, considerable progress has been made in identifying mitophagic adaptors or the degradation of parental mitochondria after fertilization and understanding the overall importance of mitophagy for aging and neurodegenerative diseases.
Mitochondrial depolarization can occur naturally during mitochondrial fission or can be induced through cellular stress pathways, including apoptosis. Upon extensive damage, mitochondrial membranes can be permeabilized through distinct routes, and mitochondrial membrane permeabilization (MMP) constitutes one of the hallmarks of apoptotic or necrotic cell death.Citation41 However, if the mitochondrial insult is not too severe and only a fraction of the mitochondrial pool is damaged, mitophagic degradation could rescue the cell and prevent cell death.
Damaged mitochondria can be recognized through the voltage-sensitive kinase Pink1. Under normal circumstances, Pink1 is continuously degraded on mitochondria, but upon loss of Δψm, Pink1 is stabilized on the outer mitochondrial membrane.Citation42–Citation44 The rapid accumulation of Pink1 on the mitochondrial surface facilitates recruitment of Parkin,Citation45–Citation49 an E3 ligase to mitochondria, where it ubiquitinates multiple mitochondrial proteins, including the fusion proteins Mfn1/2 and the VDAC1 protein.Citation6,Citation50–Citation55 The accumulation of ubiquitin-modifications is thought to facilitate the recruitment of the autophagy adaptor p62, eventually leading to the autophagosomal degradation of the damaged mitochondrion.Citation4–Citation6,Citation56 Mutations in the genes coding for both PINK1 and Parkin were identified in the early-onset forms of Parkinson disease. In cell culture models, disease-associated mutants of Pink1 and Parkin dramatically reduced the recruitment of Parkin to damaged mitochondria and their subsequent degradation.Citation5,Citation6,Citation43,Citation44,Citation52
Another protein linked to mitophagy in mammalian cells is NIX. In immature red blood cells, NIX mediates the mitophagic removal of excess mitochondria.Citation38,Citation39,Citation57 But the elimination of damaged mitochondria seems also be NIX-dependent in some cell lines.Citation58 In addition to mitophagy-mediators, the loss of general autophagy regulators, like ATG5 or ATG7, also leads to significant accumulation of damaged mitochondria,Citation59–Citation65 further supporting the idea that autophagy plays an essential role in mitochondrial quality control to ensure the health of the mitochondrial pool.
Recent studies demonstrated that mitochondria are not only a downstream substrate of mitophagy, but that they are able to actively influence their own fate during starvation-induced autophagy. Two recent studies showed that during starvation, mitochondria react to the depletion of nutrients (especially nitrogen sources) with rapid and extensive mitochondrial tubulation.Citation66,Citation67 The formation of elongated mitochondrial networks appears to be dependent on the PKA-mediated inactivation of the fission protein Drp1, removing the counterbalancing force to fusion. Interestingly, these mitochondrial networks resulted in sustained mitochondrial ATP production, enhanced cellular survivalCitation66 and, most importantly, prevented the elimination of mitochondria.Citation66,Citation67 In contrast to this, fusion-incompetent mitochondria were heavily degraded during starvation. This suggests that mitochondrial morphology actively influences mitophagic responses.
The exact mechanism by which mitochondrial fusion prevents mitophagy is still unclear. The mitochondrial size alone could be a determining factor, as the loss of Drp1-activity also decreases mitophagy under basal conditionsCitation32 and upon external mitochondrial damage.Citation4,Citation53 Alternatively, changes in mitochondrial activity and/or recruitment of mitophagy-adaptors (like Parkin) could be causative for the decreased degradation of fused mitochondria.
Mitochondrial depolarization/fragmentation are two common prerequisites for mitophagy, and mitochondrial fusion can block mitophagy. This intimate link between mitochondrial shape and degradation suggests that both processes could also be coupled on the molecular level. Indeed, two different systems were identified that affect both mitophagy and mitochondrial shape. Parkin has been suggested to regulate mitochondrial fusion in addition to its well-established function as a mitophagy adaptor.Citation4,Citation68,Citation69 A similar connection has been suggested for the autophagy-regulating proteins ATG12 and ATG3. During the induction of autophagy, ATG12 gets covalently linked to ATG5, thus driving the expansion and formation of the autophagosome. A recent study by Debnath and colleagues identified ATG3 as a further acceptor for ATG12.Citation70 Lack of the covalent ATG12/3 complex led to mitochondrial fragmentation and loss of mitophagy, partially mimicking the effects of Parkin depletion in mammalian cells. Even though mitochondrial dynamics and mitophagy are linked by several means, it will be important to understand which processes/proteins directly affect mitochondrial dynamics and which effects on mitochondrial shape are only secondary to changes in mitophagy and/or the accumulation of dysfunctional mitochondria.
Mitochondria as Autophagic Membrane Source
To date, the membrane origin(s) of autophagosomes remains under debate. Several organelles have been suggested to contribute lipids for the formation of autophagosomes, including the ER, the Golgi and plasma membrane.Citation71–Citation73 In a recent study, Hailey et al. added mitochondria to the growing list of potential autophagosomal membrane sources.Citation8,Citation74 Impressive imaging analysis revealed that during starvation, the membranes of autophagosomes and mitochondria are in continuity, allowing the transfer of a mitochondrial outer membrane marker (GFPcb5MitoTM) into nascent autophagosomes.
Although the question of how and from which organelle(s) autophagosomes originate is not fully clear, several lines of evidence support the role of mitochondria during autophagosomal biogenesis under starvation. In mammals, the autophagyregulating proteins Beclin1 and Bcl-2 not only localize to the ER and but also to mitochondria. Beclin1 is part of the pro-autophagic class III PI3K complex, which is implicated in the initiation of autophagosomal biogenesis.Citation75–Citation78 It was believed that the initiation of Beclin1-dependent autophagy is solely regulated on the level of the ER,Citation79 but studies by Cecconi and colleagues identified Ambra1 (activating molecule in beclin1-regulated autophagy) as a potential contributor of Beclin1-dependent autophagosome formation from mitochondria.Citation80 Under nutrient-rich conditions, Bcl-2 interacts both with AMBRA1 at the mitochondrial surface and Beclin1 at the ER to inhibit autophagy. But upon starvation, endogenous Ambra1 dissociates from Bcl-2, leading to an increased interaction between Ambra1 with Beclin1 on mitochondria and the ER. The mitochondrial Ambra1/Beclin1 complex could thus drive autophagsomal biogenesis from mitochondrial and ER membranes.
Another protein that could couple mitochondria to autophagosomal biogenesis is Endophilin B1, a membrane-shaping protein with pro-autophagic activity.Citation81–Citation84 Under normal circumstances, Endophilin B1 cycles between the cytosol and mitochondria and can be enriched on mitochondrial surfaces upon stress.Citation85 On mitochondria, Endophilin B1 could activate the Beclin1-PI3K complex through binding of the Beclin1 adaptor UVRAGCitation82 and drive autophagosomal biogenesis using mitochondrial membranes.
Several lines of evidence support mitochondria as a potential autophagosomal membrane source, which raises the question of why specifically starvation-induced autophagy (but not other types of autophagy) uses the mitochondrial membrane.Citation8 The lipid requirements of autophagy could contribute to this selectivity. Phosphatidyl-ethanolamine, the lipid needed to covalently link ATG8 homologs to autophagosomal membranes, can be produced in two different locales, the ER and mitochondria.Citation86 PE produced in the mitochondria is synthesized through decarboxylation of Phosphatidylserine, transferred from the ER into mitochondria. In the ER, the Kennedy pathway utilizes DAG and exogenous ethanolamine for the formation of PE. Nutrient depletion could limit the availability of DAG/ethanolamine (produced following growth factor engagement) and affect PE availability in the ER. This would make the mitochondrial membrane the primary site of PE production and, thereby, the site of autophagosomal biogenesis.
Unicellular organisms continuously adapt to fluctuating nutrient availability in their environment, but in mammalian tissues, nutrient levels are kept rather stable. Even though nutrient availability is tightly regulated, several processes that lead to the starvation of cells or tissues in mammals are known. Newborns proceed through starvation periods shortly after birth, during which autophagy is essential for survival.Citation87 But starvation-induced autophagy also plays a role in cancer. In the microenvironment of tumors, where access to nutrients and oxygen is restricted, autophagy promotes tumor cell survival.Citation88 In contrast to its pro-survival role in established tumors, autophagy suppresses tumor development. Dysfunctional mitochondria and protein aggregates are linked to reactive oxygen species (ROS) generation, activation of DNA damage and cell death.Citation89–Citation92 Degradation of these materials could protect cells against progressive cell damage, inflammation and thus cancer while promoting healthy aging.Citation93–Citation96
Therefore, mitochondria may be pivotal for autophagy in cancer, with changes in mitochondrial function regulating the autophagic process, resulting in promotion of tumor formation and/or preservation.
Mitochondria as Regulators of Autophagic Flux
Mitochondria are powerful metabolic organelles, producing precursors for lipid and amino acid synthesis, energy and signaling molecules, like reactive oxygen species (ROS). The central autophagy-regulating pathways, AMPK and mTOR, are controlled by energy levels.Citation97–Citation106 Loss of mitochondrial ATP production can thus induce autophagy in an mTOR/AMPK-dependent manner.Citation100,Citation107–Citation109 Interestingly, mTOR and AMPK not only respond to mitochondrial output but also regulate mitochondrial function and biogenesis.Citation110–Citation112 For example, the pharmacological inhibition of mTOR rapidly affects mitochondrial metabolism and decreases oxidative phosphorylation.Citation111 This clearly demonstrates the tight bidirectional connection between autophagy and mitochondria through signaling networks, which could play a central role for lifespan extension and age-related disorders.Citation95
An additional mitochondrial product that influences autophagy is ROS. During nutrient starvation, an increase in H2O2levels is essential for autophagy induction.Citation113 The autophagy protein ATG4 was identified as the basis for the redox sensitivity of autophagy. Throughout the formation of an autophagosome, the mammalian homologs of ATG8, LC3A or LC3B, are covalently attached to the nascent autophagosome, driving its expansion. The cysteine protease ATG4 can counteract the autophagosomal expansion through the removal of the ATG8 homologs from the autophagosomal membrane. ATG4 can be regulated by oxidation of a cysteine residue near its catalytic domain, which inhibits ATG4's cleavage activity and allows ATG8-mediated autophagosome expansion to proceed. Thus far, ATG4 is the only autophagy protein known to be regulated by ROS signaling, but other proteins might further contribute to the redox regulation of the autophagic process, including the autophagy master regulator mTOR itself.Citation92,Citation95,Citation114,Citation115
A recent study in yeast further expands the regulatory potential of mitochondria on the autophagic pathway. During specific starvation conditions, it was shown that mitochondrial integrity influences autophagic initiaion and/or autophagosomal degradation on the other side.Citation7 The authors identified the mitochondrial membrane potential, but not ATP production, as regulator of autophagic flux. Interestingly, mitochondrial depolarization and loss of oxygen consumption have also been linked to reduced turnover of autophagosomes in mammalian cells (Rambold AS and Lippincott-Schwartz JL, unpublished results).Citation9 Whereas mitochondrial dysfunction seemed to inhibit the recruitment of autophagy-initiation kinase ATG1/13 to the phagosomal initiation site in yeast, mitochondrial dysfunction has been linked with reduced lysosomal acidification in mammalian cells.Citation7,Citation9 The exact mechanism by which mitochondrial dysfunction influences autophagic flux still remains to be established in both systems.
Clearly, several mitochondrial outputs can regulate autophagy, either through targeting the autophagy machinery itself or autophagy regulating signaling pathways. Mitochondrial control of mTOR (through ATP and ROS)Citation100,Citation107–Citation109,Citation115 is of particular interest, as mTOR regulates a range of essential cellular functions, including protein translation and autophagy, and has been linked to aging in lower eukaryotes.Citation116 Accumulating evidence suggests that mTOR also influences aging in mammals, and that the triad between mitochondria, mTOR and autophagy (all being able to regulate one another) might present an integral regulatory node during aging and thus age-related diseases.Citation92,Citation95,Citation96,Citation115,Citation117
Mitochondria in the Autophagy: Apoptosis Crosstalk
Mitochondria play an essential role during apoptosis. Extensive cellular stress can lead to MMP, release of pro-apoptotic molecules, activation of caspases and, finally, apoptotic cell death. However, if MMP is limited to only a subset of mitochondria, this will result in the selective autophagosomal elimination of the depolarized mitochondria. This suggests efficient autophagic recognition of dysfunctional mitochondria could set a higher threshold for MMP to set an irreparable or deadly event in motion.
It is interesting to note the induction of autophagy and apoptosis are partially regulated by the same proteins. The antiapoptotic protein Bcl-2 regulates autophagy and apoptosis through binding of the pro-autophagic protein Beclin1 and the pro-apoptotic protein Bax (and others).Citation79,Citation118 Cellular stress can lead to the release of both proteins. Nutrient depletion first leads to the release of Beclin1, causing the activation of PI3K and induction of autophagy.Citation79,Citation119,Citation120 Upon extended nutrient deprivation the pro-apoptotic protein Bax is also released from Bcl-2,Citation120 allowing it to induce apoptosis. The molecular coupling of both events, autophagy and apoptosis, could suggest that the cellular response to stress is determined by the severity and longevity of the insult. In an alternative to the stress-severity model, the different subcellular localizations of Bcl-2 (ER and mitochondria) might be integral to the fate switch between autophagy and apoptosis. While the Beclin1 sequestration seems to be mainly controlled through ER-localized Bcl-2,Citation79 apoptosis could be regulated through Bcl-2 on mitochondria. Independent of the exact mechanism, it is clear that the health of mitochondria might determine the cellular outcome: autophagy or apoptosis. Thus, mitochondrial damage could overwhelm to pro-survival autophagy pathway and direct the cell toward release of pro-apoptotic Bax from Bcl-2 to induce cell death.
There are a number of additional mechanisms that couple autophagic demise to apoptotic onset involving mitochondria. The autophagy-regulating proteins Beclin1, the class III PI3K and ATG4D can all be cleaved by caspases upon which they translocate to mitochondria, where they acquire new functions and can amplify mitochondria-mediated apoptosis.Citation121,Citation122 In addition to this, caspase-dependent cleavage destroys the pro-autophagic function of Beclin1 and PI3K. Another protein that links apoptosis and autophagy is ATG5.Citation123 Upon autophagy induction, ATG5 enables the extension of the autophagosome at its site of biogenesis. However, in response to high cellular stress levels, ATG5 can be cleaved by calpains. The ATG5-cleavage product translocates to mitochondria, where it binds Bcl-XL. In contrast to cleaved Beclin1, class III PI3K or ATG4D, calpain-processed ATG5 is able to induce apoptosis without the need for additional pro-apoptotic stimuli. These data exemplify the importance of mitochondrial integrity and their localized protein networks throughout the regulation of autophagy.
Conclusion
Mitochondria have been primarily connected to cellular ATP production and metabolism but have recently begun to take center stage in many other cellular processes. Mitochondria's connection to the autophagosomal system in particular has garnered much interest in recent years. To date, several lines of evidence support the notion that mitochondria are autophagic substrates and can also shape the autophagic response in several ways. The localization of many autophagic regulators on mitochondria, the integration of mitochondria in several signaling networks and their potential to modulate these pathways all suggest a powerful mitochondrial influence on autophagy. That said, we are still at the beginning of understanding the impact mitochondria have on autophagy. Delineating the multiple factors that underlie disorders that depend on autophagy, including neurodegenerative (like Parkinson disease) inflammatory diseases or cancer, and in particular, clarifying the tight link between cancer-associated changes in mitochondrial metabolism and dependence on autophagy during different steps of tumor formation and preservation will be invaluable for devising new ways to treat and prevent these diseases.
Figures and Tables
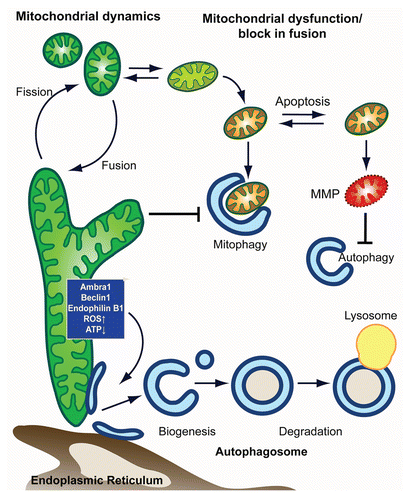
Figure 1 Model for mitochondria-autophagy crosstalk. In this model, we depict the main intersection between in autophagy-mitochondrial crosstalk from the side of (1) autophagy and (2) mitochondria. (1) Autophagy shapes mitochondrial health and number through the selective degradation of mitochondria in a process termed mitophagy. Elimination of damaged mitochondria is facilitated by mitochondrial fission and promotes cell survival. Mitophagic malfunction leads to the accumulation of dysfunctional mitochondria and makes the cell more susceptible to MMP and apoptosis. When cell death is induced, apoptotic executers inactivate pro-autophagic proteins, thus inhibiting autophagy. (2) Autophagic degradation of mitochondria is affected by mitochondrial shape/function, with heavily fused mitochondria being a poor substrate that evades autophagic degradation. Mitochondria, furthermore, are able to control autophagic induction and autophagsomal biogenesis from mitochondria (or other autohagosomal origins such as the er) through mitochondrial localized proteins and/or metabolic products (such as ROS and ATP).
Acknowledgments
We would like to thank Brenda Kostelecky and Tim Lammermann for critically reading the manuscript. We apologize for those studies we could not include in this review due to space constrains. A.S.R. was supported through a postdoctoral fellowship of the German Research Foundation (DFG).
References
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 2009; 10:458 - 467; PMID: 19491929; http://dx.doi.org/10.1038/nrm2708
- Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 2010; 22:124 - 131; PMID: 20034776; http://dx.doi.org/10.1016/j.ceb.2009.11.014
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008; 451:1069 - 1075; PMID: 18305538; http://dx.doi.org/10.1038/nature06639
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 2008; 183:795 - 803; PMID: 19029340; http://dx.doi.org/10.1083/jcb.200809125
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, et al. PINK1/Parkinmediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 2010; 12:119 - 131; PMID: 20098416; http://dx.doi.org/10.1038/ncb2012
- Geisler S, Holmstrom KM, Treis A, Skujat D, Weber SS, Fiesel FC, et al. The PINK1/Parkin-mediated mitophagy is compromised by PD-associated mutations. Autophagy 2010; 6:871 - 878; PMID: 20798600; http://dx.doi.org/10.4161/auto.6.7.13286
- Graef M, Nunnari J. Mitochondria regulate autophagy by conserved signalling pathways. EMBO J 2011; 30:2101 - 2114; PMID: 21468027; http://dx.doi.org/10.1038/emboj.2011.104
- Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 2010; 141:656 - 667; PMID: 20478256; http://dx.doi.org/10.1016/j.cell.2010.04.009
- Las G, Sereda S, Wikstrom JD, Twig G, Shirihai OS. Fatty acids suppress autophagic turnover in {beta}-cells. J Biol Chem 2011; 286:42534 - 42544; PMID: 21859708
- Hoppins S, Nunnari J. The molecular mechanism of mitochondrial fusion. Biochim Biophys Acta 2009; 1793:20 - 26; PMID: 18691613; http://dx.doi.org/10.1016/j.bbamcr.2008.07.005
- Lackner LL, Nunnari JM. The molecular mechanism and cellular functions of mitochondrial division. Biochim Biophys Acta 2009; 1792:1138 - 1144; PMID: 19100831
- Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol 2010; 191:1141 - 1158; PMID: 21149567; http://dx.doi.org/10.1083/jcb.201007152
- Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci USA 2009; 106:11960 - 11965; PMID: 19617534; http://dx.doi.org/10.1073/pnas.0904875106
- Soubannier V, McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta 2009; 1793:154 - 170; PMID: 18694785; http://dx.doi.org/10.1016/j.bbamcr.2008.07.008
- Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S. MARCH-V is a novel mitofusin 2- and Drp1binding protein able to change mitochondrial morphology. EMBO Rep 2006; 7:1019 - 1022; PMID: 16936636; http://dx.doi.org/10.1038/sj.embor.7400790
- Park YY, Lee S, Karbowski M, Neutzner A, Youle RJ, Cho H. Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J Cell Sci 2010; 123:619 - 626; PMID: 20103533; http://dx.doi.org/10.1242/jcs.061481
- Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A, et al. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem 2006; 281:37972 - 37979; PMID: 17003040; http://dx.doi.org/10.1074/jbc.M606059200
- Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol 2007; 178:757 - 764; PMID: 17709430; http://dx.doi.org/10.1083/jcb.200704112
- Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J 2006; 25:2966 - 2977; PMID: 16778770; http://dx.doi.org/10.1038/sj.emboj.7601184
- Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential and Yme1L. J Cell Biol 2007; 178:749 - 755; PMID: 17709429; http://dx.doi.org/10.1083/jcb.200704110
- Busch KB, Bereiter-Hahn J, Wittig I, Schagger H, Jendrach M. Mitochondrial dynamics generate equal distribution but patchwork localization of respiratory Complex I. Mol Membr Biol 2006; 23:509 - 520; PMID: 17127623; http://dx.doi.org/10.1080/09687860600877292
- Jakobs S. High resolution imaging of live mitochondria. Biochim Biophys Acta 2006; 1763:561 - 575; PMID: 16750866; http://dx.doi.org/10.1016/j.bbamcr.2006.04.004
- Jakobs S, Schauss AC, Hell SW. Photoconversion of matrix targeted GFP enables analysis of continuity and intermixing of the mitochondrial lumen. FEBS Lett 2003; 554:194 - 200; PMID: 14596939; http://dx.doi.org/10.1016/S0014-5793(03)01170-0
- Karbowski M, Arnoult D, Chen H, Chan DC, Smith CL, Youle RJ. Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol 2004; 164:493 - 499; PMID: 14769861; http://dx.doi.org/10.1083/jcb.200309082
- Nakada K, Inoue K, Ono T, Isobe K, Ogura A, Goto YI, et al. Inter-mitochondrial complementation: Mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nat Med 2001; 7:934 - 940; PMID: 11479626; http://dx.doi.org/10.1038/90976
- Ono T, Isobe K, Nakada K, Hayashi JI. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet 2001; 28:272 - 275; PMID: 11431699; http://dx.doi.org/10.1038/90116
- Twig G, Graf SA, Wikstrom JD, Mohamed H, Haigh SE, Elorza A, et al. Tagging and tracking individual networks within a complex mitochondrial web with photoactivatable GFP. Am J Physiol Cell Physiol 2006; 291:176 - 184; PMID: 16481372; http://dx.doi.org/10.1152/ajpcell.00348.2005
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 2006; 25:3900 - 3911; PMID: 16874299; http://dx.doi.org/10.1038/sj.emboj.7601253
- Gomes LC, Scorrano L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim Biophys Acta 2008; 1777:860 - 866; PMID: 18515060; http://dx.doi.org/10.1016/j.bbabio.2008.05.442
- Malena A, Loro E, Di Re M, Holt IJ, Vergani L. Inhibition of mitochondrial fission favours mutant over wild-type mitochondrial DNA. Hum Mol Genet 2009; 18:3407 - 3416; PMID: 19561330; http://dx.doi.org/10.1093/hmg/ddp281
- Suen DF, Narendra DP, Tanaka A, Manfredi G, Youle RJ. Parkin overexpression selects against a deleterious mtDNA mutation in heteroplasmic cybrid cells. Proc Natl Acad Sci USA 2010; 107:11835 - 11840; PMID: 20547844; http://dx.doi.org/10.1073/pnas.0914569107
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 2008; 27:433 - 446; PMID: 18200046; http://dx.doi.org/10.1038/sj.emboj.7601963
- Amchenkova AA, Bakeeva LE, Chentsov YS, Skulachev VP, Zorov DB. Coupling membranes as energy-transmitting cables. I. Filamentous mitochondria in fibroblasts and mitochondrial clusters in cardiomyocytes. J Cell Biol 1988; 107:481 - 495; PMID: 3417757; http://dx.doi.org/10.1083/jcb.107.2.481
- Aon MA, Cortassa S, O'Rourke B. Percolation and criticality in a mitochondrial network. Proc Natl Acad Sci USA 2004; 101:4447 - 4452; PMID: 15070738; http://dx.doi.org/10.1073/pnas.0307156101
- Frieden M, James D, Castelbou C, Danckaert A, Martinou JC, Demaurex N. Ca(2+) homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. J Biol Chem 2004; 279:22704 - 22714; PMID: 15024001; http://dx.doi.org/10.1074/jbc.M312366200
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1 and Opa1 in apoptosis. Mol Biol Cell 2004; 15:5001 - 5011; PMID: 15356267; http://dx.doi.org/10.1091/mbc.E04-04-0294
- Skulachev VP. Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci 2001; 26:23 - 29; PMID: 11165513; http://dx.doi.org/10.1016/S0968-0004(00)01735-7
- Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature 2008; 454:232 - 235; PMID: 18454133; http://dx.doi.org/10.1038/nature07006
- Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci USA 2007; 104:19500 - 19505; PMID: 18048346; http://dx.doi.org/10.1073/pnas.0708818104
- Sato M, Sato K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science 2011; http://dx.doi.org/10.1126/science.1210333
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 2007; 87:99 - 163; PMID: 17237344; http://dx.doi.org/10.1152/physrev.00013.2006
- Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 2010; 191:933 - 942; PMID: 21115803; http://dx.doi.org/10.1083/jcb.201008084
- Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 2010; 189:211 - 221; PMID: 20404107; http://dx.doi.org/10.1083/jcb.200910140
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 2010; 8:1000298; PMID: 20126261; http://dx.doi.org/10.1371/journal.pbio.1000298
- Kim PK, Hailey DW, Mullen RT, Lippincott-Schwartz J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc Natl Acad Sci USA 2008; 105:20567 - 20574; PMID: 19074260; http://dx.doi.org/10.1073/pnas.0810611105
- Sha D, Chin LS, Li L. Phosphorylation of parkin by Parkinson disease-linked kinase PINK1 activates parkin E3 ligase function and NFkappaB signaling. Hum Mol Genet 2010; 19:352 - 363; PMID: 19880420; http://dx.doi.org/10.1093/hmg/ddp501
- Shiba K, Arai T, Sato S, Kubo S, Ohba Y, Mizuno Y, et al. Parkin stabilizes PINK1 through direct interaction. Biochem Biophys Res Commun 2009; 383:331 - 335; PMID: 19358826; http://dx.doi.org/10.1016/j.bbrc.2009.04.006
- Um JW, Stichel-Gunkel C, Lubbert H, Lee G, Chung KC. Molecular interaction between parkin and PINK1 in mammalian neuronal cells. Mol Cell Neurosci 2009; 40:421 - 432; PMID: 19167501; http://dx.doi.org/10.1016/j.mcn.2008.12.010
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA 2010; 107:378 - 383; PMID: 19966284; http://dx.doi.org/10.1073/pnas.0911187107
- Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet 2011; 20:1726 - 1737; PMID: 21296869; http://dx.doi.org/10.1093/hmg/ddr048
- Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 2010; 19:4861 - 4870; PMID: 20871098; http://dx.doi.org/10.1093/hmg/ddq419
- Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation and HDAC6dependent mitophagy. J Cell Biol 2010; 189:671 - 679; PMID: 20457763; http://dx.doi.org/10.1083/jcb.201001039
- Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, et al. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol 2010; 191:1367 - 1380; PMID: 21173115; http://dx.doi.org/10.1083/jcb.201007013
- Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci USA 2010; 107:5018 - 5023; PMID: 20194754; http://dx.doi.org/10.1073/pnas.0913485107
- Ziviani E, Whitworth AJ. How could Parkin-mediated ubiquitination of mitofusin promote mitophagy?. Autophagy 2010; 6; PMID: 20484985; http://dx.doi.org/10.4161/auto.6.5.12242
- Okatsu K, Saisho K, Shimanuki M, Nakada K, Shitara H, Sou YS, et al. p62/SQSTM1 cooperates with Parkin for perinuclear clustering of depolarized mitochondria. Genes Cells 2010; 15:887 - 900; PMID: 20604804
- Mortensen M, Ferguson DJ, Simon AK. Mitochondrial clearance by autophagy in developing erythrocytes: clearly important, but just how much so?. Cell Cycle 2010; 9:1901 - 1906; PMID: 20495377; http://dx.doi.org/10.4161/cc.9.10.11603
- Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, et al. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem 2010; 285:27879 - 27890; PMID: 20573959; http://dx.doi.org/10.1074/jbc.M110.119537
- Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab 2008; 8:325 - 332; PMID: 18840363; http://dx.doi.org/10.1016/j.cmet.2008.08.009
- Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, et al. Autophagy is required to maintain muscle mass. Cell Metab 2009; 10:507 - 515; PMID: 19945408; http://dx.doi.org/10.1016/j.cmet.2009.10.008
- Mortensen M, Ferguson DJ, Edelmann M, Kessler B, Morten KJ, Komatsu M, et al. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci USA 2010; 107:832 - 837; PMID: 20080761; http://dx.doi.org/10.1073/pnas.0913170107
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 2007; 13:619 - 624; PMID: 17450150; http://dx.doi.org/10.1038/nm1574
- Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med 2007; 204:25 - 31; PMID: 17190837; http://dx.doi.org/10.1084/jem.20061303
- Stephenson LM, Miller BC, Ng A, Eisenberg J, Zhao Z, Cadwell K, et al. Identification of Atg5-dependent transcriptional changes and increases in mitochondrial mass in Atg5-deficient T lymphocytes. Autophagy 2009; 5:625 - 635; PMID: 19276668; http://dx.doi.org/10.4161/auto.5.5.8133
- Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 2010; 6; PMID: 20431347; http://dx.doi.org/10.4161/auto.6.5.11947
- Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 2011; 13:589 - 598; PMID: 21478857; http://dx.doi.org/10.1038/ncb2220
- Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA 2011; 108:10190 - 10195; PMID: 21646527; http://dx.doi.org/10.1073/pnas.1107402108
- Kuroda Y, Mitsui T, Kunishige M, Shono M, Akaike M, Azuma H, et al. Parkin enhances mitochondrial biogenesis in proliferating cells. Hum Mol Genet 2006; 15:883 - 895; PMID: 16449237; http://dx.doi.org/10.1093/hmg/ddl006
- Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lammermann K, et al. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem 2009; 284:22938 - 22951; PMID: 19546216; http://dx.doi.org/10.1074/jbc.M109.035774
- Radoshevich L, Murrow L, Chen N, Fernandez E, Roy S, Fung C, et al. ATG12 conjugation to ATG3 regulates mitochondrial homeostasis and cell death. Cell 2010; 142:590 - 600; PMID: 20723759; http://dx.doi.org/10.1016/j.cell.2010.07.018
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol-3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 2008; 182:685 - 701; PMID: 18725538; http://dx.doi.org/10.1083/jcb.200803137
- Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol 2010; 12:747 - 757; PMID: 20639872; http://dx.doi.org/10.1038/ncb2078
- Young AR, Chan EY, Hu XW, Kochl R, Crawshaw SG, High S, et al. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci 2006; 119:3888 - 3900; PMID: 16940348; http://dx.doi.org/10.1242/jcs.03172
- Rambold AS, Lippincott-Schwartz J. Starved cells use mitochondria for autophagosome biogenesis. Cell Cycle 2010; 9:3633 - 3634; PMID: 20855967; http://dx.doi.org/10.4161/cc.9.18.13170
- Furuya N, Yu J, Byfield M, Pattingre S, Levine B. The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Autophagy 2005; 1:46 - 52; PMID: 16874027; http://dx.doi.org/10.4161/auto.1.1.1542
- Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol-3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol 2001; 152:519 - 530; PMID: 11157979; http://dx.doi.org/10.1083/jcb.152.3.519
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999; 402:672 - 676; PMID: 10604474; http://dx.doi.org/10.1038/45257
- Tassa A, Roux MP, Attaix D, Bechet DM. Class III phosphoinositide-3-kinase-Beclin1 complex mediates the amino acid-dependent regulation of autophagy in C2C12 myotubes. Biochem J 2003; 376:577 - 586; PMID: 12967324; http://dx.doi.org/10.1042/BJ20030826
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005; 122:927 - 939; PMID: 16179260; http://dx.doi.org/10.1016/j.cell.2005.07.002
- Strappazzon F, Vietri-Rudan M, Campello S, Nazio F, Florenzano F, Fimia GM, et al. Mitochondrial BCL-2 inhibits AMBRA1-induced autophagy. EMBO J 2011; 30:1195 - 1208; PMID: 21358617; http://dx.doi.org/10.1038/emboj.2011.49
- Pierrat B, Simonen M, Cueto M, Mestan J, Ferrigno P, Heim J. SH3GLB a new endophilin-related protein family featuring an SH3 domain. Genomics 2001; 71:222 - 234; PMID: 11161816; http://dx.doi.org/10.1006/geno.2000.6378
- Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol 2007; 9:1142 - 1151; PMID: 17891140; http://dx.doi.org/10.1038/ncb1634
- Takahashi Y, Meyerkord CL, Wang HG. BARgaining membranes for autophagosome formation: Regulation of autophagy and tumorigenesis by Bif-1/Endophilin B1. Autophagy 2008; 4:121 - 124; PMID: 18032918
- Takahashi Y, Meyerkord CL, Wang HG. Bif-1/endophilin B1: a candidate for crescent driving force in autophagy. Cell Death Differ 2009; 16:947 - 955; PMID: 19265852; http://dx.doi.org/10.1038/cdd.2009.19
- Karbowski M, Jeong SY, Youle RJ. Endophilin B1 is required for the maintenance of mitochondrial morphology. J Cell Biol 2004; 166:1027 - 1039; PMID: 15452144; http://dx.doi.org/10.1083/jcb.200407046
- Vance JE. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res 2008; 49:1377 - 1387; PMID: 18204094; http://dx.doi.org/10.1194/jlr.R700020-JLR200
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature 2004; 432:1032 - 1036; PMID: 15525940; http://dx.doi.org/10.1038/nature03029
- Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res 2011; 17:654 - 666; PMID: 21325294; http://dx.doi.org/10.1158/1078-0432.CCR-10-2634
- Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation and tumorigenesis. Cancer Cell 2006; 10:51 - 64; PMID: 16843265; http://dx.doi.org/10.1016/j.ccr.2006.06.001
- Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell 2009; 137:1062 - 1075; PMID: 19524509; http://dx.doi.org/10.1016/j.cell.2009.03.048
- Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev 2007; 21:1367 - 1381; PMID: 17510285; http://dx.doi.org/10.1101/gad.1545107
- Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY) 2009; 1:425 - 437; PMID: 20157526
- Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008; 456:259 - 263; PMID: 18849966; http://dx.doi.org/10.1038/nature07416
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006; 441:885 - 889; PMID: 16625204; http://dx.doi.org/10.1038/nature04724
- Morselli E, Galluzzi L, Kepp O, Criollo A, Maiuri MC, Tavernarakis N, et al. Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol. Aging (Albany NY) 2009; 1:961 - 970; PMID: 20157579
- Raimundo N, Shadel GSA. “radical” mitochondrial view of autophagy-related pathology. Aging (Albany NY) 2009; 1:354 - 356; PMID: 20157522
- Egan D, Kim J, Shaw RJ, Guan KL. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 2011; 7:643 - 644; PMID: 21460621; http://dx.doi.org/10.4161/auto.7.6.15123
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011; 331:456 - 461; PMID: PMID:21205641; http://dx.doi.org/10.1126/science.1196371
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 2008; 30:214 - 226; PMID: 18439900; http://dx.doi.org/10.1016/j.molcel.2008.03.003
- Inoki K, Zhu T. Guan KL, TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003; 115:577 - 590; PMID: 14651849; http://dx.doi.org/10.1016/S0092-8674(03)00929-2
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011; 13:132 - 141; PMID: 21258367; http://dx.doi.org/10.1038/ncb2152
- Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS ONE 2010; 5:15394; PMID: 21072212; http://dx.doi.org/10.1371/journal.pone.0015394
- Löffler AS, Alers S, Dieterle AM, Keppeler H, Franz-Wachtel M, Kundu M, et al. Ulk1-mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. Autophagy 2010; 7:696 - 706; PMID: 21460634; http://dx.doi.org/10.4161/auto.7.7.15451
- Shang L, Chen S, Du F, Li S, Zhao L, Wang X. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc Natl Acad Sci USA 2011; 108:4788 - 4793; PMID: 21383122; http://dx.doi.org/10.1073/pnas.1100844108
- Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr 2011; 93:891 - 896; PMID: 21325438; http://dx.doi.org/10.3945/ajcn.110.001925
- Hardie DG. AMP-activated protein kinase: a cellular energy sensor with a key role in metabolic disorders and in cancer. Biochem Soc Trans 2011; 39:1 - 13; PMID: 21265739; http://dx.doi.org/10.1042/BST0390001
- Desai BN, Myers BR, Schreiber SL. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc Natl Acad Sci USA 2002; 99:4319 - 4324; PMID: 11930000; http://dx.doi.org/10.1073/pnas.261702698
- Schieke SM, Phillips D, McCoy JP Jr, Aponte AM, Shen RF, Balaban RS, et al. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem 2006; 281:27643 - 27652; PMID: 16847060; http://dx.doi.org/10.1074/jbc.M603536200
- Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature 2010; 466:68 - 76; PMID: 20562859; http://dx.doi.org/10.1038/nature09204
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 2007; 450:736 - 740; PMID: 18046414; http://dx.doi.org/10.1038/nature06322
- Ramanathan A, Schreiber SL. Direct control of mitochondrial function by mTOR. Proc Natl Acad Sci USA 2009; 106:22229 - 22232; PMID: 20080789; http://dx.doi.org/10.1073/pnas.0912074106
- Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol 2006; 574:33 - 39; PMID: 16709637; http://dx.doi.org/10.1113/jphysiol.2006.109512
- Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 2007; 26:1749 - 1760; PMID: 17347651; http://dx.doi.org/10.1038/sj.emboj.7601623
- Filomeni G, Desideri E, Cardaci S, Rotilio G, Ciriolo MR. Under the ROS…thiol network is the principal suspect for autophagy commitment. Autophagy 2010; 6:999 - 1005; PMID: 20639698; http://dx.doi.org/10.4161/auto.6.7.12754
- Sarbassov DD, Sabatini DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem 2005; 280:39505 - 39509; PMID: 16183647; http://dx.doi.org/10.1074/jbc.M506096200
- Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet 2008; 24:604 - 612; PMID: 18992957; http://dx.doi.org/10.1016/j.tig.2008.10.002
- Hands SL, Proud CG, Wyttenbach A. mTOR's role in aging: protein synthesis or autophagy?. Aging (Albany NY) 2009; 1:586 - 597; PMID: 20157541
- Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 2011; 18:571 - 580; PMID: 21311563; http://dx.doi.org/10.1038/cdd.2010.191
- Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell 2008; 30:678 - 688; PMID: 18570871; http://dx.doi.org/10.1016/j.molcel.2008.06.001
- Wei Y, Sinha S, Levine B. Dual role of JNK1mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy 2008; 4:949 - 951; PMID: 18769111
- Betin VM, Lane JD. Caspase cleavage of Atg4D stimulates GABARAP-L1 processing and triggers mitochondrial targeting and apoptosis. J Cell Sci 2009; 122:2554 - 2566; PMID: 19549685; http://dx.doi.org/10.1242/jcs.046250
- Wirawan E, Vande Walle L, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis 2010; 1:18; PMID: 21364619; http://dx.doi.org/10.1038/cddis.2009.16
- Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol 2006; 8:1124 - 1132; PMID: 16998475; http://dx.doi.org/10.1038/ncb1482