 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chronic chagasic cardiomyopathy is a leading cause of heart failure in Latin American countries, being associated with intense inflammatory response and fibrosis. We have previously shown that bone marrow mononuclear cell (BMC) transplantation improves inflammation, fibrosis, and ventricular diameter in hearts of mice with chronic Chagas disease. Here we investigated the transcriptomic recovery induced by BMC therapy by comparing the heart transcriptomes of control, chagasic, and BMC transplanted mice. Out of the 9390 unique genes quantified in all samples, 1702 had their expression altered in chronic chagasic hearts compared to those of normal mice. Major categories of significantly upregulated genes were related to inflammation, fibrosis and immune responses, while genes involved in mitochondrion function were downregulated. When BMC-treated chagasic hearts were compared to infected mice, 96% of the alterations detected in infected hearts were restored to normal levels, although an additional 109 genes were altered by treatment. Transcriptomic recovery, a new measure that considers both resotrative and side effects of treatment, was remarkably high (84%). Immunofluorescence and morphometric analyses confirmed the effects of BMC therapy in the pattern of inflammatory-immune response and expression of adhesion molecules. In conclusion, by using large-scale gene profiling for unbiased assessment of therapeutic efficacy we demonstrate immunomodulatory effects of BMC therapy in chronic chagasic cardiomyopathy and identify potentially relevant factors involved in the pathogenesis of the disease that may provide new therapeutic targets.
Introduction
The discovery of stem cells with potential therapeutic properties to promote tissue regeneration recovery has opened new avenues for the treatment of degenerative and traumatic disorders, including heart failure. Patients with Chagas disease, one of the main causes of heart failure in Latin American countries, could possibly benefit from a stem cell-based therapy aiming to ameliorate the heart function deteriorated by the chronic infection with Trypanosoma cruzi. Currently, heart transplantation is the only efficient therapy for chagasic patients with heart failure, a procedure limited by the scarcity of donated organs and complications when performed in T. cruzi-infected patients due to the risk of a resurgence of parasitemia and tissue parasitism upon administration of immunosuppressive drugs. Therefore, instead of replacing the hearts of chagasic heart failure individuals, the perspective of decreasing the damage of the heart and restoring pre-infection status with cell-based therapies presents an attractive option that should be investigated.Citation1
Chronic chagasic cardiomyopathy is a progressive disease characterized by focal or disseminated inflammation causing myocytolysis, necrosis and progressive deposition of collagen.Citation2 Transplantation of bone marrow cells (BMC) was reported to reduce inflammation and fibrosis in the heart. The decrease in inflammatory cells correlated with an increase in apoptotic cells in the inflammatory infiltrate.Citation3 A regression of right ventricular dilation, typically observed in a specific chagasic mouse model, was observed in mice treated with BMC, as detected by magnetic resonance image analysis.Citation4 In addition, administration of granulocyte-colony stimulating factor (G-CSF), a cytokine capable of mobilizing BMCs to the peripheral blood, to chagasic mice caused a decrease in inflammation and fibrosis and improvement of cardiopulmonary function.Citation5 Altogether, these results suggest a paracrine effect of BMC therapy, modulating the immune response that causes tissue aggression and fibrosis deposition in the heart.Citation1
The identification of the mechanisms by which BMCs modulate the immune-inflammatory response and promote improvement of cardiac damage in infected mice may contribute to the development of more efficient therapies for chagasic patients. We have previously shown that a number of genes related to inflammation and fibrosis are altered in the hearts of chagasic mice compared to normal controls.Citation6 In this study we have used cDNA microarray analysis as an unbiased, high throughput approach to evaluate the efficacy of transplantation of BMC to restore the normal transcriptome in the myocardium of mice chronically infected with T. cruzi. Our results show a marked transcriptomic recovery caused by the BMC therapy in the hearts of mice with chronic chagasic cardiomyopathy and suggest that such an analytical approach may be useful to evaluate efficacy of other treatments for various pathological conditions.
Results
Injection of BMCs in mice with chronic chagasic cardiomyopathy reduces inflammation and fibrosis.
Mice infected with the Colombian strain of T. cruzi develop a progressive myocarditis accompanied by fibrosis during the chronic phase of infection. Treatment with BMCs caused a decrease in the number of inflammatory cells () and a reduction in fibrosis () compared to saline-treated controls. An intense multifocal inflammatory response composed mainly by mononuclear cells, associated with myocytolysis, was observed in sections of saline-treated chagasic mice (). Smaller and less frequent foci of inflammatory cells were found in heart sections of chagasic mice treated with BMCs ().
Gene regulation by BMC transplantation.
We compared transcriptomic changes in the BMC-treated and untreated infected mouse hearts with respect to the uninfected hearts, defining as significantly regulated those genes whose mean expression level changed by more than 1.5-fold and that were different at the 0.05 level of significance when the variation among the four biological replicas was considered. Microarray results have been deposited in www.ncbi.nlm.nih.gov/gds as GSE24088. Two months after transplantation of BMC (at eight months after infection), 9,390 genes were quantifiable on all twelve arrays (from uninfected controls, infected and saline-treated and BMC treated infected hearts); of these, 480 (5.12%) were downregulated and 1,222 (13.01%) were upregulated in the infected saline-treated hearts. GenMapp analysis was used to determine whether regulated genes encoding proteins performing specific functions were differentially affected. Of the upregulated genes, most prominent Gene Ontology (GO) terms that corresponded to these pathways were immune system process/immune response, chemokine receptor binding and chemokine activity, including inflammatory response and chemotaxis genes Ccl12, Ccl6, Ccl7, Ccl8 (50-fold upregulated), Ccl9 (40-fold upregulated) and Cxcl1. Downregulated genes in the infected hearts were most prominently associated with mitochondria (cofactor binding, TCA cycle, glycoloysis, oxidative phsophorylation, coenzyme biosynthesis, oxydoreductase activity, electron transport, NADH, RNA biosynthesis), although other affected pathways included axon guidance receptor (Ephb3, Q8c419), negative regulation of BMP signaling (Htra1, Twsg1, Flt1), transmembrane receptor tyrosine kinase activity (Erbb3, Ephb3, Q8c419), cell cycle arrest (Ak1, Cdkn1b, Cdkn1c, Sesn1) and multidrug transport (Slc47a1).
In the infected hearts of mice treated with BMC, we found that expression of 103 genes (1.21% of the quantified genes) were upregulated compared to controls and 77 (0.91%) were downregulated. Most prominent categories of upregulated genes were those associated with angiogenesis and blood vessel development (Casp8, Col18a1, Dicer1, Myh9, Pdgfa, Tnfrsf12a, Cxcl12, Fgf8, Serpinf1, Stab1, Btg1, Notch1) and endothelial cell development (Vezf1), thiamin transport and folate carrier activity (Slc19a2) and vitamin biosynthesis (Itgb1bp3), cyclin binding (Cdkn1a) and purine catabolism (Ampd2). Downregulated gene categories included nuclear transport (Rangrf, Akt1), cofactor metabolic process (Akt1, Ndufs1, Ldhd, Acss1, Idh3a, Sdha, Sucla2, Suclg2, Coq5, Coq6, Coq9) and isomerase activity (flbp10, hsd367, Fkpb10, Hsd3b7, Tpi1), calcium ion binding (Eef2k, Fkbp10, Frem2) and cell cycle arrest (Ak1, Cdkn1b). The remarkable restoration of the control gene expression pattern by BMC therapy of infected mice is summarized in and is quantified by the TRE score, calculated as follows:
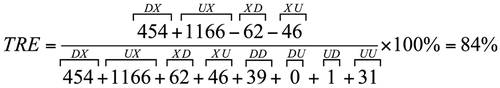
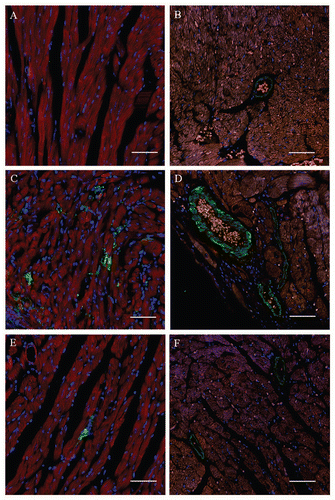
Alterations in the expression pattern of galectin-3 and syndecan-4 in hearts of mice with chronic chagasic cardiomyopathy after BMC transplantation.
The expression of two proteins encoded by genes upregulated by T. cruzi infection and modulated by BMC therapy was investigated by confocal analysis. Galectin-3 was found highly expressed in macrophages of the inflammatory infiltrate in chagasic hearts, when compared to non-infected controls (, and C). Upon BMC treatment, the number of galectin-3 positive cells was significantly reduced in heart sections of chagasic mice ( and ). Syndecan-4, a heparan-sulfate proteoglycan that regulates cell-matrix interactions and is present in focal adhesions, was found highly expressed in endothelial cells in chagasic hearts compared to normal mice ( and D). Heart sections of BMC-treated mice showed a significant reduction in the intensity of syndecan-4 staining and in the number of syndecan-4+ blood vessels (, C and ). In contrast, the total number of blood vessels was similar in the three groups, as indicated by staining with antibodies against von Willebrand Factor ().
Discussion
Stem cell transplantation has become an attractive therapeutic possibility for patients with cardiac diseases, including chronic chagasic cardiomyopathy. The demonstration that BMC transplantation has beneficial effects in hearts of mice with chronic chagasic cardiomyopathy has raised several questions and the determination of the molecular and cellular mechanisms by which these cells exert their action may contribute to the development of new therapeutic strategies for chronic chagasic cardiomyopathy based on cell transplantation and/or cell factors. Here we performed a microarray analysis in an attempt to understand some of the mechanisms involved in the activity of BMC in the mouse model of chronic chagasic cardiomyopathy. Gene expression analysis was carried out comparing hearts of mice with chronic chagasic cardiomyopathy treated or not with BMC and non-infected controls. We have found that most of the genes altered by infection are modulated by BMC therapy, indicating a dramatic effect of these cells in the mechanisms of pathogenesis of the disease. The use of large-scale gene profiling for unbiased assessment of therapeutic efficacy is thus a major contribution of the present work to cell based therapies.
Previously we have shown that a significant number of genes with expression altered in the heart by infection with the Colombian T. cruzi strain are related to inflammation and fibrosis.Citation6 As expected, since BMC therapy causes a significant decrease in inflammation and fibrosis, we found that most of these upregulated genes in chronic chagasic cardiomyopathy have their expression in the heart decreased after cell therapy.
The decrease in inflammation observed two months after BMC therapy (at a total of eight months post-infection) was previously associated with an increased number of apoptotic cells compared to untreated chagasic controls.Citation3 In contrast to the high number of inflammatory cells undergoing apoptosis during the indeterminate stage,Citation7 a reduced number (about 0.5%) of cells in the inflammatory infiltrate were found undergoing apoptosis in hearts of individuals with chronic chagasic cardiomyopathy.Citation8 We found several genes related to apoptosis altered by T. cruzi infection and modulated by BMC therapy. Analysis such as the one used in this study will detect global alterations in gene expression, representing all cell types present in the heart, including cardiomyocytes and inflammatory cells. Thus, additional studies are needed to clarify which mechanisms are involved in the prevention of apoptosis of inflammatory cells in chronic chagasic cardiomyopathy, as well as those promoting apoptosis after BMC therapy.
The inflammatory infiltrate present in hearts of mice with chronic chagasic cardiomyopathy is mainly composed by mononuclear cells found adhered to myofibers, many of them in process of myocytolysis.Citation2 Macrophages are one of the main populations found in the inflammatory site, and are highly activated by IFNγ and TNFα, two cytokines produced in the hearts of chagasic mice.Citation6 Here we found that the expression of galectin-3 (also known as Mac-2), a member of a large family of lectins that are highly conserved throughout animal evolution, upregulated in chagasic mice, is modulated by BMC therapy. By immuno-fluorescence analysis, we showed that this molecule is expressed mainly in macrophages within the inflammatory infiltrate in the hearts of chagasic mice. A previous study has demonstrated, in a model of hypertrophied heart in rats, that galectin-3 was the most overexpressed gene in failing versus functionally compensated hearts.Citation9 Galectin-3 colocalized with activated myocardial macrophages and treatment of rats with recombinant galectin-3 induced cardiac fibroblast proliferation, collagen production, cyclin D1 expression and left ventricular dysfunction.Citation9 In addition, galectin-3 is known to play important roles in inflammatory responses, including suppression of T-cell apoptosis and its expression is induced by IFNγ in macrophages found in inflammatory infiltrates in the heart.Citation10,Citation11 Altogether, these data suggest an important role of galectin-3 in the pathogenesis of chronic chagasic cardiomyopathy, and indicate this molecule as a target for development of new treatments for chronic chagasic cardiomyopathy. In fact, galectin-3 production has recently been pointed out as a novel marker of heart failure in patients.Citation12
Another molecule with its gene expression modulated by BMC therapy was syndecan-4, a heparin sulfate-carrying cell surface protein expressed by a number of different cell types, including endothelial cells, smooth muscle cells and cardiac myocytes that participates in processes of cell signaling, adhesion and migration.Citation13,Citation14 Little is known about the regulation of syndecan-4, but it has been reported to increase after various forms of tissue injury including vascular wall injury,Citation13 or myocardial infarction.Citation14 Syndecan-4 expression has been shown to be increased with migration of blood-derived macrophages after myocardial infarction.Citation14 Zhang et al. (1999) have shown that TNFα is the principal factor produced by the ischemic myocytes responsible for induction of endothelial cell syndecan-4 expression.Citation15 To our knowledge, this is the first study investigating the expression of syndecan-4 in Chagas disease. The fact that TNFα levels are increased in chronic chagasic hearts may explain the finding of enhanced expression of syndecan-4 observed herein.Citation6 Furthermore, the modulation of the chronic inflammatory response in chagasic hearts induced by BMC therapy may explain the reduction of syndecan-4 expression found in BMC-treated animals.
A correlation between intensity of expression of syndecan-4 in endothelial cells was found in close association with inflammatory infiltrates, but this was not due to an increase in the number of vWF+ blood vessels. The microarray analysis suggested an increase in angiogenesis in hearts of chronic chagasic mice after BMC therapy, but this was not confirmed by the immunohistochemistry analysis. This apparent disparity may be explained by the fact that the genes upregulated after BMC therapy, participate in a number of other biological processes in addition to those related to blood vessel formation, such as apoptosis.Citation16–Citation18
We previously commented on the prominent upregulation of immune response genes in the chagasic heart and validated substantial numbers of these gene products using ELISA, real-time PCR and confocal microscopy.Citation6 The identification of mitochondria-associated genes as one of the pathways that are most downregulated in the infected heart is entirely consistent with reports by Garg and others indicating that mitochondrial function is a prominent target of infection in acute and chronic states.Citation19–Citation21
Gene expression profiling with microarrays has been widely used to characterize tissue and cell responses to various stimuli, to identify disease biomarkers and to reveal components and interplay within and between gene regulatory networks. The application of this unbiased high throughput method to compare a pathological situation with changes occurring after a therapeutic regimen here revealed a striking degree of recovery of control gene expression status in infected mice treated with BMC. As we previously showed in a study examining structural and physiological parameters of hearts of chagasic mice, the BMC therapy not only prevents further damage over time but also reverses the damage that was present before the stem cells were injected.Citation4
In conclusion, this unbiased global gene expression analysis indicated a potent restorative effect of BMC in the hearts of mice with chronic chagasic cardiomyopathy, with over 90% recovery of normal expression level of genes altered by T. cruzi infection. This impressive effect is most probably mediated by a paracrine effect through the secretion of soluble mediators. From this global expression profile identification of such factors is possible and may lead to the association or the replacement of cell therapy by these cellular hormones in order to achieve the desired repair to the damaged chagasic heart.
Materials and Methods
Animals.
Four week-old female and male C57Bl/6 mice were used for T. cruzi infection. All animals, weighing 20–23 g, were raised and maintained at the Goncalo Moniz Research Center/FIOCRUZ in rooms with controlled temperature (22 ± 2°C) and humidity (55 ± 10%) and continuous air flow. Animals were housed in a 12 h light/12 h dark cycle (6 am–6 pm) and provided with rodent diet and water ad libitum. Animals were handled according to the NIH guidelines for animal experimentation. All procedures described had prior approval from the local IACUC (Albert Einstein College of Medicine and Fiocruz, Bahia).
Infection with Trypanosoma cruzi.
Trypomastigotes of the myotropic Colombian T. cruzi strain were obtained from culture supernatants of infected LCC-MK2 cells. Infection of C57Bl/6 mice was performed by intraperitoneal injection of 1,000 T. cruzi trypomastigotes in saline. Parasitemia of infected mice was evaluated at various times after infection by counting the number of trypomastigotes in peripheral blood aliquots.
Bone marrow cell (BMC) transplantation.
BMCs obtained from femurs and tibiae of C57Bl/6 mice were used in transplantation experiments.Citation3,Citation4 Briefly, BMCs were purified by centrifugation in Ficoll gradient at 1,000 g for 15 minutes (Histopaque 1119 and 1077, 1:1; Sigma, St. Louis, MO). After two washings in RPMI medium (Sigma), the cells were resuspended in saline, filtered over nylon wool and injected intravenously in chagasic mice (3 ç 106 cells/mouse) six months after infection. Non-transplanted control mice received intravenous injections of the same volume (200 µl) of saline.
Morphometric analysis.
Groups of mice were sacrificed at two months after BMC or saline injection (at eight months after infection) and hearts removed and fixed in 10% buffered formalin. Heart sections were analyzed by light microscopy after paraffin embedding, followed by standard hematoxylin/eosin staining. Inflammatory cells infiltrating heart tissue were counted using a digital morphometric evaluation system. Images were digitized using a color digital video camera adapted to a microscope. The images were analyzed using the Image Pro Program (Media Cybernetics, San Diego, CA), such that the inflammatory cells were counted and integrated with respect to area. Ten fields per section were counted in 5–10 sections per heart. The percentage of fibrosis was determined using Sirius red-stained heart sections and the Image Pro Plus v.7.0 Software to integrate the areas.
Immunofluorescence.
Frozen or formalin-fixed paraffin embedded hearts were sectioned and 4 µm-thick sections were used for detection of galectin-3 and syndecan-4 expression by immunofluorescence. First, paraffin embedded sections were deparaffinizated and a heat-induced antigen retrieval step in citrate buffer (pH = 6.0) was performed. Then, sections were incubated overnight with the following primary antibodies: anti-galectin-3, 1:50 (Santa Cruz Biotechnology), anti-syndecan-4, 1:50 (Santa Cruz Biotechnology) or anti-vWF, 1:100 (Dako). On the following day, sections were incubated with Alexa fluor 633 conjugated Phalloidin, 1:50, mixed with one of the secondary antibodies Alexa fluor 488-conjugated anti-goat IgG, 1:200 or Alexa fluor 488-conjugated anti-rabbit IgG, 1:200 (Molecular Probes) for 1 hour. Nuclei were stained with 4,6-diamidino-2-phenylindole (VectaShield Hard Set mounting medium with DAPI H-1500; Vector Laboratories). The presence of fluorescent cells was determined by observation on a FluoView 1000 confocal microscope (Olympus). Approximately 10 random fields per animal were captured using a 40× objective. Morphometric analyses were performed using Image Pro Plus v.7.0 software.
DNA microarray and data analysis.
We compared RNA samples extracted from whole hearts of 4 control, 4 chagasic and 4 BMC-treated chagasic mice by analyzing hybridization to MO30k microarrays printed by Duke University (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL8938) spotted with 70-mer oligonucleotides (mouse Operon version 3.0). The hybridization protocol has already been described in reference Citation6, the slide type and the scanner settings were uniform throughout the entire experiment to minimize the technical noise. Briefly, 20 µg total RNA extracted in Trizol from each of the twelve samples (individual hearts) was reverse transcribed in the presence of fluorescent Alexa Fluor® 555- and Alexa Fluor®647-aha-dUTPs (Invitrogen, Carlsbad, CA) to obtain labeled cDNA. Red and green labeled samples of biological replicas were then co-hybridized (“multiple yellow” strategyCitation22) overnight at 50°C. After washing (0.1% SDS and 1% SSC) to remove the non-hybridized cDNA, each array was scanned at 630 V (635 nm) and 580 V (532 nm) with GenePix 4100B scanner (Axon Instruments, Union City, CA) and images were primarily analyzed with GenePixPro 6.0 (Molecular Devices, Sunnyvale, CA). Microarray data were processed as described previously in reference 6. A gene was considered as significantly up or downregulated when comparing four hearts from one condition to those from another if the absolute fold change was >1.5× and the p-vlaue of the Student's heteroscedastic t-test of equality of the means of the distributions with a Bonferronitype adjustment for each redundancy group (set of spots probing the same gene) was <0.05. GenMappCitation23 and MappFinder (www.genmapp.org) software and associated databases were used to identify the most affected GO (Gene Ontology) categories. In order to determine whether genes differentially expressed in untreated and treated infected hearts with respect to uninfected hearts were disproportionately affected in specific pathways, we used GenMAPP and MappFinder software (Gladstone Institute; San Francisco: www.genmapp.org) to provide the statistics of affected GO categories. GO sets with fewer than 10 analyzed members were excluded from this analysis.
The novel parameter transcriptomic recovery efficacy (TRE) was computed as:
where: D, U, X indicate whether the gene was down, up or not regulated in the saline-treated (first position) or BMC-treated (second position) infected hearts. TRE is thus the percentage of up and downregulated genes in infected hearts whose expression level was restored to normal (UX & DX) penalized by the percentage of not regulated genes in infected hearts whose expression changed due to treatment alone (XD & XU).
Statistical analysis.
Morphometric data were analyzed using Student's t test or ANOVA followed by Turkey. Differences were considered significant when p < 0.05.
Abbreviations
| BMC | = | bone marrow cell |
| G-CSF | = | granulocyte-colony stimulator factor |
| GO | = | gene ontology |
| vWF | = | von willebrand factor |
Figures and Tables
Figure 1 Transplantation of BMC decreases inflammation and fibrosis in hearts of C57Bl/6 mice chronically infected with Colombian strain T. cruzi. Mice were infected with 1,000 trypomastigote forms of Colombian strain T. cruzi. Inflammation (A) and fibrosis (B) were quantified in heart sections of normal mice, mice 8 months after infection injected with saline (Saline) or with bone marrow cells (BMC), stained with H&E and Sirius red. Bars represent the means ± SEM of 5–8 animals/group at six months after infection. *p < 0.05; ***p < 0.001. Heart sections of chagasic mice injected with saline (C) or with BMC (D), stained with H&E (original magnification ×40).
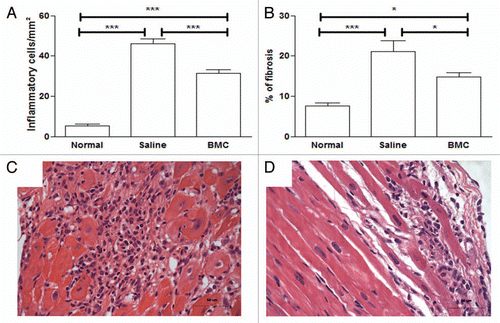
Figure 2 Significantly regulated genes in BMC-untreated (injected with saline) (A) and BMC-treated (B) infected hearts with respect to controls. A substantial reduction in the number of regulated genes was observed in treated hearts. Significantly regulated immune response genes in untreated (vertical axis) and treated (horizontal axis) (C). Symbols of certain regulated genes were written close to their representative points. Note that, while the upregulation of ifitm1 and psca was reduced by treatment, the regulation of all other immune response gene was fully recovered. However, lfi2712a and lfi2712b, whose expression was not significantly altered by the infection, were found as downregulated in treated hearts.
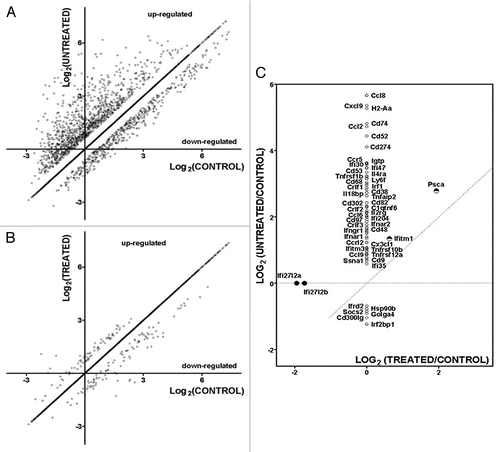
Figure 3 Quantification of galectin-3, syndecan-4 and vWF. Expression of galectin-3 (A), syndecan-4 (B and C) and vWF (D) were quantified by immunohistochemistry and morphometric analysis in heart sections of normal or eight month chagasic mice that had been injected at six months after infection with saline (Saline) or with bone marrow cells (BMC). Bars represent the means ± SEM of 3 animals/group. *p < 0.05; **p < 0.01; ***p < 0.001.
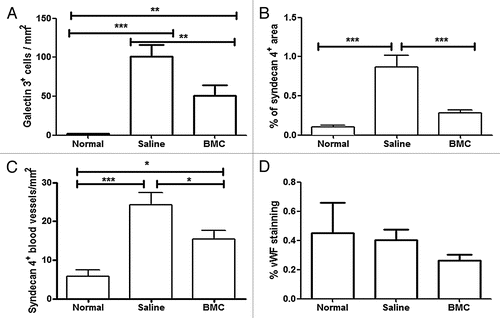
Figure 4 Expression of galectin-3 and syndecan-4 in hearts of chronic chagasic mice treated with bone marrow cells. Heart sections of normal mice (A and B), of mice injected with saline (C and D) or with BMC (E and F). Sections were stained (red) with anti-galectin-3 (A, C and E) or anti-syndecan-4 (B, D and F). Nuclei (blue) were stained with DAPI. Scale bars: 50 µm (original magnification ×40).
Acknowledgments
Financial support for these studies was provided by the National Institutes of Health (HL-73732, HD-32573, AI-076248), CAPES, CNPq, FINEP, FAPERJ and FAPESB. R.C.S.G. and L.R. were supported by a Fogarty International Training Grant D43TW007129. The authors thank Carine Machado for technical assistance.
References
- Soares MBP, Santos RR. Current status and perspectives of cell therapy in Chagas disease. Mem Inst Oswaldo Cruz 2009; 104:325 - 332
- Köberle F. Chagas disease and Chagas syndromes: the pathology of American Trypanosomiasis. Adv Parasitol 1968; 6:63 - 116
- Soares MB, Lima RS, Rocha LL, Takyia CM, Pontes-de-Carvalho L, de Carvalho AC, et al. Transplanted bone marrow cells repair heart tissue and reduce myocarditis in chronic chagasic mice. Am J Pathol 2004; 164:441 - 447
- Goldenberg RC, Jelicks LA, Fortes FSA, Weiss LM, Rocha LL, Zhao D, et al. Bone Marrow Cell Therapy Ameliorates and Reverses chagasic cardiomyopathy in a mouse model. J Infec Dis 2008; 197:544 - 547
- Macambira SG, Vasconcelos JF, Costa C, Klein W, Lima RS, Guimarães P, et al. Granulocyte colony-stimulating factor treatment in chronic Chagas disease: Preservation and improvement of cardiac structure and function. FASEB J 2009; 23:3843 - 3850
- Soares MBP, Lima RS, Rocha LL, Vasconcelos JF, Rogatto SR, Santos RR, et al. Gene expression changes associated with myocarditis and fibrosis in hearts of mice with chronic chagasic cardiomyopathy. J Infec Dis 2010; 202:416 - 426
- Andrade ZA. Immunopathology of Chagas disease. Mem Inst Oswaldo Cruz 1999; 94:71 - 80
- Rossi MA, Souza AC. Is apoptosis a mechanism of cell death of cardiomyocytes in chronic chagasic myocarditis?. Int J Cardiol 1999; 68:325 - 331
- Sharma UC, Pokharel S, Brakel TJV, Berlo JHV, Cleutjens JPM, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004; 110:3121 - 3128
- Rabinovich GA, Ramhorst RE, Rubinstein N, Corigliano A, Daroqui MC, Kier-Joffé EB, et al. Induction of allogenic T-cell hyporesponsiveness by galectin-1-mediated apoptotic and non-apoptotic mechanisms. Cell Death Differ 2002; 9:661 - 670
- Reifenberg K, Lehr HA, Torzewski M, Steige G, Wiese E, Küpper I, et al. Interferon-gamma induces chronic active myocarditis and cardiomyopathy in transgenic mice. Am J Pathol 2007; 171:463 - 472
- Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, et al. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the DEAL-HF study. Clin Res Cardiol 2010; 99:323 - 328
- Nikkari ST, Jarvelainen HT, Wight TN, Ferguson M, Clowes AW. Smooth muscle cell expression of extracellular matrix genes after arterial injury. Am J Pathol 1994; 144:1348 - 1356
- Li J, Brown LF, Laham RJ, Volk R, Simons M. Macrophage-dependent regulation of syndecan gene expression. Circ Res 1997; 81:785 - 796
- Zhang Y, Pasparakis M, Kollias G, Simons M. Myocyte-dependent regulation of endothelial cell syndecan-4 expression. Role of TNFalpha. J Biol Chem 1999; 274:14786 - 14790
- Zhao Y, Sui X, Ren H. From procaspase-8 to caspase-8: Revisiting structural functions of caspase-8. J Cell Physiol 2010; 225:316 - 320
- Pelus LM, Horowitzc D, Coopera SC, King AG. Peripheral blood stem cell mobilization: A role for CXC chemokines. Crit Rev Oncol Hematol 2002; 43:257 - 275
- Berg JS, Powell BC, Cheney RE. A Millennial Myosin Census. Mol Biol Cell 2001; 12:780 - 794
- Garg N, Popov VL, Papaconstantinou J. Profiling gene transcription reveals adeficiency of mitochondrial oxidative phosphorylation in Trypanosoma cruzi-infected murine hearts: Implications in chagasic myocarditis development. Biochim Biophys Acta 2003; 1638:106 - 120
- Gupta S, Wen JJ, Garg NJ. Oxidative Stress in Chagas Disease. Interdiscip Perspect Infect Dis 2009; 2009:1 - 8
- Wen JJ, Yachelini PC, Sembaj A, Manzur RE, Garg NJ. Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Radic Biol Med 2006; 41:270 - 276
- Iacobas DA, Iacobas S, Urban-Maldonado M, Scemes E, Spray DC. Similar transcriptomic alterations in Cx43 knock-down and knock-out astrocytes. Cell Commun Adhes 2008; 15:195 - 206
- Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat Genet 2002; 31:19 - 20