Abstract
Salmonella typhimurium double leu-arg auxotrophs have been shown to be highly effective as antitumor agents in nude mouse models of human metastatic cancer. In order to proceed to clinical development of the S. typhimurium double auxotroph, termed A1-R, it is necessary to evaluate antitumor efficacy in immunocompetent mice. In the present study, we have observed the efficacy of A1-R on the Lewis lung (LLC) carcinoma in vitro as well as in C57BL/6 (C57) immunocompetent mice. In vitro, A1-R treatment of LLC began to induce cell death within one hour. Various doses and schedules of A1-R were administered to C57 mice implanted with LLC, including bolus single intravenous injection; medium dose with weekly intravenous administration and metronomic treatment with small intravenous doses twice a week. Bolus treatment was toxic to the immunocompetent host, in contrast to nude mice. Lower-dose weekly doses and metronomic doses were well tolerated by the immunocompetent host. Weekly intravenous injection with 2 x 107 bacteria and twice a week intravenous injection with 107 bacteria significantly inhibited metastasis formation, while bolus injection was toxic. Intra-thoracic administration was carried out with 108 bacteria A1-R injected into Lewis lung-bearing C57 mice weekly for three weeks. Lung metastasis was significantly inhibited by intrathoracic bacterial administration, without toxicity. The results in this report, demonstrating the anti-metastatic efficacy of S. typhimurium A1-R in immunocompetent mice, indicate the clinical potential of bacterial therapy of cancer.
Introduction
We have previously reported a genetically modified bacteria strain, Salmonella typhimurium A1, selected for anticancer activity in vivo. S. typhimurium A1 is auxotrophic (leu/arg-dependent). 1 The strain grows in tumor xenografts. In sharp contrast, normal tissue is cleared of these bacteria even in immunodeficient athymic mice. In vitro, the GFP-expressing bacteria grew in the cytoplasm of PC-3 human prostate cancer cells and caused nuclear destruction. These effects were visualized in cells labeled with GFP in the nucleus and red fluorescent protein in the cytoplasm. In vivo, the bacteria caused tumor inhibition and regression of tumors visualized by whole-body imaging. The bacteria, introduced i.v. or intratumorally, invaded and replicated intracellularly in PC-3 prostate cancer cells labeled with red fluorescent protein grafted into nude mice. When the bacteria were injected intratumorally, the tumor completely regressed by day 20. There were no obvious adverse effects on the host when the bacteria were injected by either route. The S. typhimurium A1 strain grew throughout the tumor, including viable malignant tissue. This result is in marked contrast to bacteria previously tried for cancer therapy that were confined to necrotic areas of the tumor, which may account, in part, for the strain's unique antitumor efficacy.Citation2–Citation9
In order to increase tumor-targeting capability of A1, the strain was re-isolated after infection of a human colon tumor growing in nude mice. The tumor-isolated strain, termed A1-R, had increased targeting for tumor cells in vivo as well as in vitro compared with A1. Treatment with A1-R resulted in highly effective tumor targeting, including viable tumor tissue and significant tumor shrinkage in mice with s.c. or orthotopic human breast cancer xerographs. Survival of the treated animals was significantly prolonged. Forty percent of treated mice were cured completely and survived as long as non-tumor-bearing mice.Citation10
A1-R was used to treat metastatic PC-3 human prostate tumors that had been orthotopically implanted in nude mice. GFP was used to image tumor and metastatic growth. Of the 10 mice with the PC-3 tumors that were injected weekly with S. typhimurium A1-R, seven were alive and well at the time the last untreated mouse died. Four A1-R-treated mice remain alive and well 6 mo after implantation. Ten additional non-tumor-bearing mice were injected weekly to determine the toxicity of S. typhimurium A1-R. No toxic effects were observed.Citation11
A new targeting strategy for primary bone tumor and lung metastasis with the modified auxotrophic strain of S. typhimurium A1-R was developed. A1-R was injected in the tail vein three times on a weekly basis. Primary bone tumor size was significantly inhibited in the treated group, and lung metastasis was almost eradicated. Therefore, A1-R treatment was effective for both primary bone tumor and lung metastasis.Citation12
A1-R was administered to an orthotopic human pancreatic tumor in nude mice. After 7 d of treatment, the pancreatic cancer had regressed without the need for chemotherapy.Citation13
A1-R was administered to both axillary lymph and popliteal lymph node metastasis of human pancreatic cancer and fibrosarcoma, respectively, as well as lung metastasis of the fibrosarcoma in nude mice. The bacteria were delivered via a lymphatic channel to target the lymph node metastases and systemically via the tail vein to target the lung metastasis. The metastases were eradicated without the need of chemotherapy or any other treatment. No adverse effects were observed.Citation14
A1-R was shown to target and inhibit the growth of liver metastasis in a mouse model of pancreatic glioma. Locally as well as systemically administered A1-R inhibited liver metastasis of pancreatic cancer. Mice treated with A1-R given locally, via intrasplenic injection, or systemically, via tail vein injection, had a much lower hepatic and splenic tumor burden compared with control mice. Systemic treatment with intravenous A1-R also increased survival time.Citation15
A1-R was administered systemically or intrathecally, to spinal cord cancer in orthotopic mouse models. Untreated mice became paralyzed due to the spinal glioma growth, and treatment with A1-R either delayed or prevented paralysis. Intrathecally treated animals had a significantly better survival than the i.v.-treated group as well as over the control group.Citation16
Leschner et al. observed a rapid increase of TNFα in blood, in addition to other pro-inflammatory cytokines, after S. typhimurium treatment of tumors. This induced a great influx of blood into the tumors by vascular disruption, and bacteria were flushed into the tumor along with the blood.Citation17
In order to further study tumor-vascular disruption by bacteria, red fluorescent protein (RFP)-expressing Lewis lung cancer cells (LLC-RFP) were transplanted subcutaneously in the ear, back skin and footpad of nestin-driven green fluorescent protein (GFP) (ND-GFP) transgenic nude mice, which selectively express GFP in nascent blood vessels. The ear tumor had more abundant blood vessels than that on the back or footpad. Tumor vascularity correlated positively with vascular disruption and tumor efficacy of A1-R, suggesting that bacteria efficacy on tumors involves vessel destruction, which depends on the extent of vascularity of the tumor.Citation18
The studies described above used nude-mouse models which are T-cell deficient. It is very important to determine the anticancer efficacy of S. typhimurium in an immunocompetent host as a bridge to the clinic. The present report demonstrates the efficacy of S. typhimurium A1-R in the Lewis lung carcinoma model in immunocompetent C57B16 mice.
Results and Discussion
Confocal imaging of lung cancer cell death induced by S. typhimurium A1-R targeting in vitro.
The interaction between S. typhimurium A1-R expressing GFP and Lewis lung carcinoma cells labeled with RFP in the cytoplasm and GFP in the nucleus, was observed with the Fluoview FV1000 confocal microscope (Olympus). GFP-expressing A1-R invaded the dualcolor Lewis lung carcinoma cells and rapidly began to induce cell death as early as 60 min after infection (). The cells appeared to die by bursting 90 min after bacteria targeting. This result showed virulence of A1-R for Lewis lung carcinoma cells ().
Lewis lung carcinoma metastasis in the lung of immunocompetent mice was targeted by S. typhimurium A1-R after systemic administration.
RFP-labeled Lewis lung carcinoma cells (1 × 106) were injected into the tail vein to obtain experimental lung metastasis in C57 mice. By day 5, there were tumor colonies in the lung (). At that time, A1-R bacteria (107 CFU) were injected into the tail vein of the C57 mice. The lungs were excised on day 2 and day 4 after A1-R administration from sacrificed mice. Tumor targeting by S. typhimurium A1-R was observed with the OV100 Small Animal Imaging System. The RFP-Lewis lung carcinoma experimental metastasis and GFP-labeled A1-R were observed in the lungs of C57 mouse on day 2 (). By day 4, A1-R grew inside the targeted RFP metastases and caused the metastases to lose their red fluorescence due to the cytotoxicity of A1-R ().
Inhibition of experimental Lewis lung carcinoma metastasis by S. typhimurium A1-R administered systemically.
RFP-labeled Lewis lung carcinoma cells (1 × 106) were injected into the tail vein of C57 mice. On day 5, there were tumor metastases in the lung. At this time, A1-R (107 CFU) was injected into the tail vein of the mice every three days. On day 15, all animals were sacrificed. The excised lungs were weighed and imaged using the OV100. Fewer RFP Lewis lung carcinoma metastases were found in the A1-R-treated lungs than in the lungs of the untreated mice. The weight of the lungs in the treated mice was significantly less than the weight of the lungs in the untreated control mice (p < 0.05). These results indicated that lung metastasis was strongly inhibited by systemic treatment with A1-R ().
Survival of C57 mice with Lewis lung carcinoma experimental metastasis is prolonged by S. typhimurium A1-R systemic administration.
RFP-labeled Lewis lung carcinoma cells (1 × 106) were injected into the tail vein of the mice. On day 5, A1-R (107 CFU) were injected into the tail vein of the mice every three days, six times. This treatment resulted in prolonged survival of the treated mice (). The average survival time of the treated mice was 36 d compared with 23 d for the untreated mice (p = 0.001).
However, the tail vein of the mice became strongly inflamed after repeated tail-vein injection of bacteria, which had to be discontinued. As a consequence, the mice in the treated group eventually died. Tail vein injection became a dose-limiting toxicity.
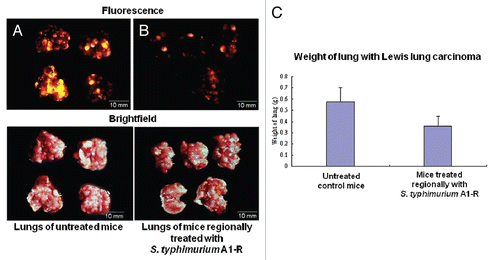
Inhibition of Lewis lung carcinoma experimental metastasis by regional administration of S. typhimurium A1-R.
Lewis lung carcinoma cells expressing RFP (1 × 106) were injected in the tail vein of C57 mice. A1-R (108 CFU) was injected into the thoracic cavity of Lewis lung carcinoma-bearing C57 mice weekly for three weeks, beginning on day 5 after cancer cell implantation. All animals were then sacrificed. The excised lungs were weighed and imaged using the OV100. RFP-labeled Lewis lung carcinoma experimental metastasis was inhibited in the A1-R-treated mice compared with the lungs of the untreated mice. The weight of lungs of the treated mice was significantly less than the weight of the lungs of the untreated mice (p < 0.05). These results indicated that lung metastasis was inhibited by regional bacteria therapy, in this case by intrathoracic administration (). No toxicity was observed.
Safety of S. typhimurium A1-R administration in C57 immunocompetent mice.
C57 mice were given a bolus treatment of 5 × 107 CFU A1-R in 100 µl PBS with a single intravenous injection, or a medium intravenous injection dose of 2 × 107 CFU for 3 weeks, or a metronomic dose of 107 CFU twice a week by intravenous administration for 3 weeks. Bolus treatment was toxic to the host. The animals showed inactivity, loss of body weight, inability to groom and ruffling of fur. Fifty percent of the mice died by day 3. The metronomic treatment was best tolerated by the mice. Animals had only slight body weight loss and had normal fur and activity.
Intrathoracic administration allowed 108 CFU A1-R to be administered to the host with no obvious toxicity with a weekly infection schedule for 3 weeks. The animals could tolerate higher doses of A1-R bacteria and more frequent administration with this route.
S. typhimurium also targets tumor vasculature.Citation17,Citation18 Suitably modified liposomesCitation19 or nano-particlesCitation20 can also target tumor vasculature which suggests combining them with S. typhimurium A1-R for even greater efficacy. Such therapies can be better followed with state-of-the-art optimal tomographyCitation21 or magnetic resonance spectroscopy based on tumor metabolism.Citation22 Lung cancer targeting by S. typhimurium A1-R may also be enhanced by other agents that target lung cancer, such as an epidermal growth factor receptor (EGFP) tyronsine kinase inhibitor.Citation23
The goal is to develop a tumor-seeking S. typhimurium strain that can kill primary and metastatic cancer without toxic effects to an immunocompetent host and without the need for combination with toxic chemotherapy. Toward this goal, S. typhimurium, A1-R was developed, which has greatly increased antitumor efficacy but maintains its original auxotrophy for leu-arg that prevents it from mounting a continuous infection in normal tissues. In the present report, A1-R was effective in monotherapy on mouse models of experimental lung metastatic cancer in an immunocompetent C57 mouse. Future studies will be aimed to bring S. typhimurium A1-R treatment of cancer to the clinic.Citation24
Materials and Methods
Preparation of bacteria.
The S. typhimurium A1-R bacteria were grown overnight on LB medium and then diluted 1:10 in LB medium. Bacteria were harvested at late-log phase, washed with PBS, and then diluted in PBS. Bacteria were then injected into the tail vein of nude mice.Citation10
Cancer cell culture.
Lewis lung carcinoma cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM glutamine (Gibco-BRL, Life Technologies, Inc.). The cell line was cultured at 37°C in a 5% incubator.
RFP vector production and transduction of the Lewis lung cancer cell line were performed as follows:Citation25–Citation27 the RFP (DsRed-2) gene (Clontech Laboratories) was inserted in the retroviral-based mammalian expression vector pLNCX (Clontech) to form the pLNCX DsRed-2 vector, as previously described in references 25–27. For RFP gene transduction, 70% confluent Lewis lung carcinoma cells were incubated with a 1:1 precipitated mixture of pLNCX DsRed-2-retroviral-containing supernatants of PT67 cells and RPMI 1640 or other culture medium (Life Technologies) containing 10% fetal bovine serum (Atlanta Biologicals) for 72 h and selected using G418 as previously described in references Citation25–Citation27.
Imaging in live mice.
The Olympus OV100 Small Animal Imaging System (Olympus), containing an MT-20 light source (Olympus) and DP70 CCD camera (Olympus), was used for imaging in live mice.Citation28 High-resolution images were captured directly on a PC (Fujitsu Siemens). Images were processed for contrast and brightness and analyzed with the use of Paint Shop Pro 8 and Cell (Olympus).
Confocal imaging of cancer cells infected with S. typhimurium A1-R in vitro.
Confocal microscopy (Fluoview FV1000, Olympus) was used for high-resolution imaging of cancer cells infected with S. typhimurium in vitro. Excitation sources included a cw semiconductor laser at 473 nm for GFP excitation and a tunable Mai Tai HP femtosecond laser emitting at 700–1,020 nm (Newport-Spectra Physics). Fluorescence images were obtained using the 20x/0.50 Uplan FLN and 40x/1.3 Oil Olympus UPLAN FLN objectives.Citation29
Experimental Lewis lung carcinoma metastasis in the lungs of C57 mice.
Female C57 immunocompetent mice, age 6 weeks, were used. RFP-expressing Lewis lung carcinoma cells (2 × 106 in 100 µl PBS) were injected into the tail vein of C57 mice.
Bacterial dosing.
For treatment of Lewis lung carcinoma cells growing in the lung, either a single high-dose (5 × 107 bacteria); a medium dose, 2 × 107 CFU per mouse, by weekly injection or a low metronomic dose, (1 × 107 CFU) were administered per mouse twice a week intravenously. 1 × 108 CFU bacteria were administered per mouse intrathoracically.
Monitoring and evaluation.
The mice were monitored weekly for signs of toxicity, including inactivity, inability to groom, ruffling of fur and body weight. Lungs were excised from lung cancer-bearing mice and were weighed and imaged.
Statistical analysis.
All statistical analyses were performed using SPSS version 12.0 (SPSS, Inc.). The experimental data are expressed as the mean ± SD. Statistical analysis using two-tailed Student t-test was used.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures and Tables
Figure 1 Lewis lung carcinoma cell death was induced by S. typhimurium A1-R targeting in vitro. Dual-color Lewis lung carcinoma cells were grown in 24-well tissue culture plates to a density of approximately 104 cells/well. S. typhimurium A1-R were grown to late log in LB broth, diluted in cell culture medium and added to the cancer cells (1 × 105/ml) and incubated at 37°C. After 40 min, the cells were rinsed and cultured in medium containing gentamycin sulfate (20 µg/ml) to kill external, but not internal, bacteria. The interaction of A1-R with tumor cells in vitro was observed with the Fluoview FV1000 confocal imaging system (Olympus).
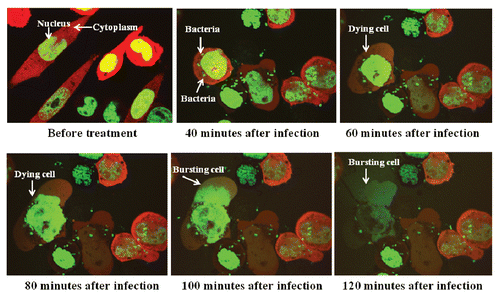
Figure 2 Lewis lung carcinoma experimental metastasis in the lung of C57 immunocompetent mice treated with i.v.-administered S. typhimurium A1-R. RFP-labeled Lewis lung carcinoma cells (1 × 106) were injected into the tail vein to obtain experimental lung metastasis in C57 mice. On day 5, S. typhimurium A1-R bacteria (107 CFU) were injected into the tail vein of C57 mice. Animals were sacrificed, and the lungs were excised on day 2 and day 4 after A1-R injection. Imaging was with the Olympus OV100. (A) RFP-labeled lung metastasis (arrow) in the C57 mouse before bacterial treatment. (B) GFP-labeled bacteria (yellow arrow) in the RFP-labeled lung metastasis (white arrow) at day 2 after i.v. administration of S. typhimurium A1-R-GFP. (C) GFP-labeled bacteria (yellow arrow) in the RFP-labeled lung metastasis (white arrow) at day 2 after S. typhimurium A1-R-GFP bacteria i.v. administration. (D and E) GFP-labeled bacteria (E) (yellow arrow) in the RFP-labeled lung metastasis (D) (white arrow) at day 4 after S. typhimurium A1-R-GFP i.v. administration. (D) is observed under an RFP filter. (E) is the same field observed under a GFP filter. (F) GFP-labeled bacteria (yellow arrow) growing in RFP-labeled lung metastasis (white arrow) at day 14 after S. typhimurium A1-R-GFP bacteria i.v. administration.
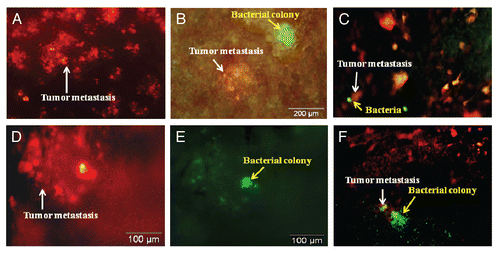
Figure 3 Efficacy of S. typhimurium A1-R treatment on RFP-labeled Lewis lung experimental lung metastasis in C57 immunocompetent mice. Female C57 immunocompetent mice, age 6 weeks, were injected with RFP-expressing Lewis lung carcinoma cells (1 × 106 in 100 µl PBS) into the tail vein. On day 5, S. typhimurium A1-R (107 CFU) were injected into the tail vein of the mice every three days. On day 15, all animals were sacrificed. The excised lungs were weighed and imaged using the Olympus OV100. (A) Fewer RFP-labeled lung metastasis were found in the lungs of mice treated with S. typhimurium A1-R-GFP than in the lungs of the untreated mice. (B) RFP-labeled lung metastasis in the lungs of the untreated C57 control mice. (C) The weight of the lungs from mice treated with S. typhimurium A1-R-GFP was significantly less than the weight of the lungs of the untreated control mouse.
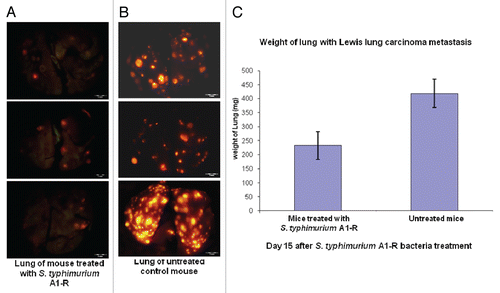
Figure 4 Survival of Lewis lung carcinoma-bearing C57 mice after S. typhimurium A1-R i.v. administration. Female C57 immunocompetent mice, aged 6 weeks, were injected with RFP-expressing Lewis lung carcinoma cells (1 × 106 in 100 µl PBS) into the tail vein. Beginning at day 5, S. typhimurium A1-R bacteria (107 CFU) were injected into the tail vein of the mice twice a week for three weeks. The survival of mice was determined for both treated and untreated groups.
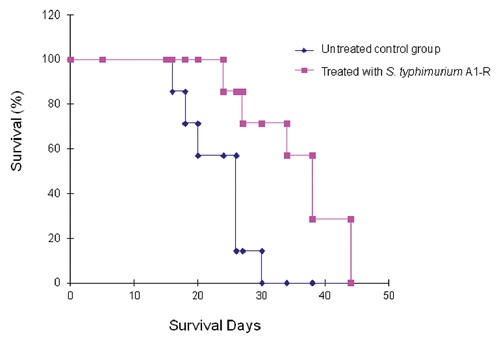
Figure 5 S. typhimurium A1-R inhibition of Lewis lung carcinoma experimental metastasis with intrathoracic bacteria administration. Female C57 immunocompetent mice, aged 6 weeks, were injected with RFP-expressing Lewis lung carcinoma cells (1 × 106 in 100 µl PBS) into the tail vein. Beginning at day 5, 1 × 108 CFU S. typhimurium were injected into thoracic cavity weekly for three weeks. All animals were sacrificed at week 4. The excised lungs were weighed and imaged using the Olympus OV100. (A) RFP-labeled metastasis in the lungs of untreated C57 control mice. (B) RFP-labeled metastasis in the lungs of mice treated by intrathoracic S. typhimurium A1-R-GFP. (C) The weight of the lungs from mice treated with intrathoracic S. typhimurium A1-R compared with the weight of the lungs from untreated mice.
Acknowledgments
This study was supported in part by the National Cancer Institute grant CA126023.
References
- Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA 2005; 102:755 - 760; PMID: 15644448; http://dx.doi.org/10.1073/pnas.0408422102
- Low KB, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, et al. Lipid A mutant Salmonella with suppressed virulence and TNFα induction retain tumor-targeting in vivo. Nat Biotechnol 1999; 17:37 - 41; PMID: 9920266; http://dx.doi.org/10.1038/5205
- Clairmont C, Lee KC, Pike J, Ittensohn M, Low KB, Pawelek J, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis 2000; 181:1996 - 2002; PMID: 10837181; http://dx.doi.org/10.1086/315497
- Sznol M, Lin SL, Bermudes D, Zheng LM, King I. Use of preferentially replicating bacteria for the treatment of cancer. J Clin Invest 2000; 105:1027 - 1030; PMID: 10772643; http://dx.doi.org/10.1172/JCI9818
- Yazawa K, Fujimori M, Amano J, Kano Y, Taniguchi S. Bifidobacterium longum as a delivery system for cancer gene therapy: Selective localization and growth in hypoxic tumors. Cancer Gene Ther 2000; 7:269 - 274; PMID: 10770636; http://dx.doi.org/10.1038/sj.cgt.7700122
- Yazawa K, Fujimori M, Nakamura T, Sasaki T, Amano J, Kano Y, et al. Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res Treat 2001; 66:165 - 170; PMID: 11437103; http://dx.doi.org/10.1023/A:1010644217648
- Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci USA 2001; 98:15155 - 15160; PMID: 11724950; http://dx.doi.org/10.1073/pnas.251543698
- Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 1981; 291:238 - 239; PMID: 7015147; http://dx.doi.org/10.1038/291238a0
- Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res 1997; 57:4537 - 4544; PMID: 9377566
- Zhao M, Yang M, Ma H, Li X, Tan X, Li S, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res 2006; 66:7647 - 7652; PMID: 16885365; http://dx.doi.org/10.1158/0008-5472.CAN-06-0716
- Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA 2007; 104:10170 - 10174; PMID: 17548809; http://dx.doi.org/10.1073/pnas.0703867104
- Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle 2009; 8:870 - 85; PMID: 19221501; http://dx.doi.org/10.4161/cc.8.6.7891
- Nagakura C, Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, et al. Efficacy of a genetically modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res 2009; 29:1873 - 1878; PMID: 19528442
- Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem 2009; 106:992 - 998; PMID: 19199339; http://dx.doi.org/10.1002/jcb.22078
- Yam C, Zhao M, Hayashi K, Ma H, Kishimoto H, McElroy M, et al. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J Surg Res 2010; 164:248 - 255; PMID: 19766244; http://dx.doi.org/10.1016/j.jss.2009.02.023
- Kimura H, Zhang L, Zhao M, Hayashi K, Tsuchiya H, Tomita K, et al. Targeted therapy of spinal cord glioma with a genetically modified Salmonella typhimurium. Cell Prolif 2010; 43:41 - 48; PMID: 19922490; http://dx.doi.org/10.1111/j.1365-2184.2009.00652.x
- Leschner S, Westphal K, Dietrich N, Viegas N, Jablonska J, Lyszkiewicz M, et al. Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNFα. PLoS ONE 2009; 4:e6692; PMID: 19693266; http://dx.doi.org/10.1371/journal.pone.0006692
- Liu F, Zhang L, Hoffman RM, Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle 2010; 9:4518 - 4524; PMID: 21135579; http://dx.doi.org/10.4161/cc.9.22.13744
- Mann AP, Bhavane RC, Somasunderam A, Liz Montalvo-Ortiz B, Ghaghada KB, Volk D, et al. Thioaptamer conjugated liposomes for tumor vasculature targeting. Oncotarget 2011; 2:298 - 304; PMID: 21666286
- Qiao Y, Huang X, Nimmagadda S, Bai R, Staedtke V, Foss CA, et al. A robust approach to enhance tumor-selective accumulation of nanoparticles. Oncotarget 2011; 2:59 - 68; PMID: 21378416
- Uchugonova A, Hoffman RM, Weinigel M, Koenig K. Watching stem cells in the skin of living mice non-invasively. Cell Cycle 2011; 10:2017 - 2020; PMID: 21558804; http://dx.doi.org/10.4161/cc.10.12.15895
- Beloueche-Babari M, Workman P, Leach MO. Exploiting tumor metabolism for non-invasive imaging of the therapeutic activity of molecularly targeted anticancer agents. Cell Cycle 2011; 10:2883 - 2893; PMID: 21857160; http://dx.doi.org/10.4161/cc.10.17.17192
- Dienstmann R, Martinez P, Felip E. Personalizing therapy with targeted agents in non-small cell lung cancer. Oncotarget 2011; 2:165 - 177; PMID: 21444946
- Wall DM, Srikanth CV, McCormick BA. Targeting tumors with Salmonella typhimurium—potential for therapy. Oncotarget 2010; 1:721 - 728; PMID: 21321381
- Hoffman RM, Yang M. Subcellular imaging in the live mouse. Nat Protoc 2006; 1:775 - 782; PMID: 17406307; http://dx.doi.org/10.1038/nprot.2006.109
- Hoffman RM, Yang M. Color-coded fluorescence imaging of tumor-host interactions. Nat Protoc 2006; 1:928 - 935; PMID: 17406326; http://dx.doi.org/10.1038/nprot.2006.119
- Hoffman RM, Yang M. Whole-body imaging with fluorescent proteins. Nat Protoc 2006; 1:1429 - 1438; PMID: 17406431; http://dx.doi.org/10.1038/nprot.2006.223
- Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H, et al. Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification wholemouse imaging system. Cancer Res 2006; 66:4208 - 4214; PMID: 16618743; http://dx.doi.org/10.1158/0008-5472.CAN-05-3927
- Uchugonova A, Duong J, Zhang N, König K, Hoffman RM. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem 2011; 112:2046 - 2050; PMID: 21465525; http://dx.doi.org/10.1002/jcb.23122