Abstract
Manzamines are a unique class of β-carboline marine alkaloids with an unusual tetra- or pentacyclic system. These alkaloids have shown a variety of bioactivities against infectious diseases, cancer and inflammatory diseases. The greatest potential for the manzamine alkaloids appears to be against malaria, with improved potency relative to chloroquine and artemisinin. Over 80 manzamine-related alkaloids have been isolated from more than 16 species of marine sponges belonging to five families distributed from the Red Sea to Indonesia, which suggests a possible microbial origin for manzamine alkaloids. The current review summarizes marine literature, focusing on the biological activities of manzamines, the possible microbial origin of this class of compounds and the Red Sea as a possible source of manzamines from biosynthetic gene clusters of Red Sea microbes.
Introducing Manzamine Alkaloids
Manzamines are a unique class of polycyclic alkaloids identified from marine sponges in the late 1980s.Citation1 These compounds possess a fused and bridged unique 5-, 6-, 6-, 8-, 13-membered heterocyclic ring system coupled to a β-carboline moiety through an apparent Pictet-Spengler reaction with the aldehyde known as ircinal A shown in the biogenetic pathway proposed by Baldwin and Whitehead.Citation2 Manzamine A, the prototype of this group, was first reported as the hydrochloride salt by Higa’s group from an Okinawan sponge Haliclona sp in 1986.Citation1
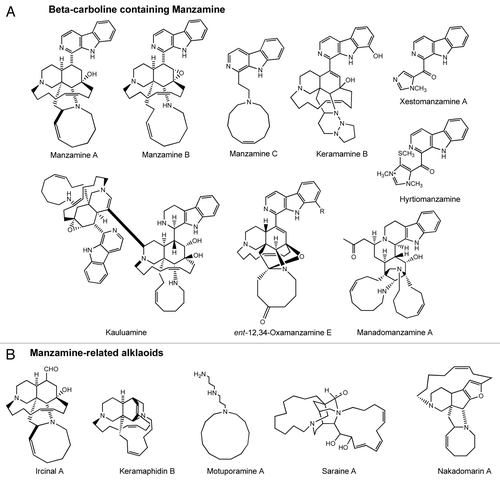
Following manzamine A, over 80 β-carboline-containing manzamine alkaloids and manzamine-related alkaloids have been isolated from marine sponges over the past two decades and the discovery of additional manzamine producing sponges seems highly likely. Wide structural variation has been reported, including the complex manzamine dimers kauluamineCitation3 and neo-kauluamine,Citation4 manadomanzamines A and B with unprecedented ring arrangementsCitation5 and 12,34-Oxamanzamines with a novel ring system generated through a new ether bridge formed between carbons 12 and 34 of the typical manzamine structureCitation6 ().
presents a survey of the β-carboline-containing manzamine and manzamine-related alkaloids discovered to date. Following this survey, it now appears that manzamines and related alkaloids are restricted to eight genera (Haliclona, Reniera, Amphimedon, Cribrochalina, Acanthostrongylophora, Petrosia, Hyrtios, Ircinia) within five families (Chalinidae, Niphatidae, Petrosiidae, Thorectidae, Irciniidae) in two orders (Haplosclerida, Dictyoceratida). These sponges have been collected from Okinawa, Philippines, Indonesia, Red Sea, Italy, South Africa and Papua New Guinea. Species producing the highest yields of manzamines are those in the genera Amphimedon spCitation12,Citation42,Citation46 and Acanthostrongylophora,Citation5,Citation6,Citation38,Citation47 which to date have yielded the greatest number of β-carboline-containing manzamine and manzamine-related alkaloids.
Table 1. Marine sponges yielding manzamines and related alkaloids
Biological Activity
The manzamine alkaloids have shown a diverse range of bioactivities including: cytotoxicity,Citation1,Citation13,Citation26,Citation27,Citation31,Citation32,Citation44,Citation48-Citation50 antimicrobial,Citation4,Citation39,Citation41 insecticidal,Citation32,Citation50 anti-inflammatoryCitation51 properties, and have shown activity against HIV and AIDS opportunistic infections.Citation52 To date, the greatest potential for the manzamine alkaloids appears to be against malaria with manzamine A, (-)-8-hydroxymanzamine A, as well as neo-kauluamineCitation13 showing improved activity over the clinically used drugs chloroquine and artemisinin in animal models. Recently, the manzamines have been patented for their immunosuppressant activity, which makes them candidates for use in organ transplant and autoimmune diseases.Citation53
The extraordinary biological activity of manzamines has stimulated great interest in finding a way to ensure a steady supply of these drug-leads for clinical trials and applications.
A simple method for kilogram-scale preparation of manzamines has been developed where the fresh sponge was extracted with acetone, and then a total alkaloid fraction was obtained using an acid-base partition scheme followed by fractionation using a silica gel vacuum liquid chromatography procedure. To overcome insufficiencies of this method, a model large-scale supercritical fluid chromatography (SFC) system for producing manzamine alkaloids was established.Citation54 As with any natural source of a potential medicine, a large-scale collection of the source marine sponge can be difficult due to scarcity of the invertebrate, and can also have negative environmental consequences. In addition, natural supplies can fluctuate, either seasonally or due to environmental changes.
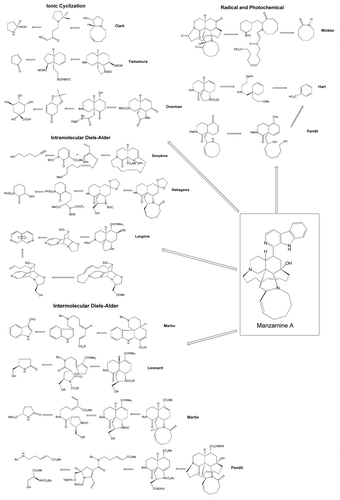
Ideally, an efficient chemical synthesis of the desired natural product could be achieved; however the structural complexity of these natural products makes them challenging targets for total synthesis.Citation55,Citation56 Synthetic studies of the manzamines have been reported by a number of groups using five different strategies ().Citation57 These include biomimetic, radical cyclisation and photochemical, ionic cyclisation, intermolecular Diels-Alder type cycloadditions, and intramolecular Diels-Alder cycloadditions approaches. Cycloadditions play a preeminent role in these synthetic studies, but alternative strategies based for instance, on [2+2] cycloaddition or Mannich cyclisations are also quite efficient. Biomimetic syntheses, however, still at an early stage, may prove to be versatile giving rise not only to manzamine type alkaloids, but also to related alkaloids in this series.Citation57
All reported syntheses to date represent inefficient multistep approaches that cannot meet the demands of pre-clinical and clinical development. Identification of a simpler, more easily synthesized structure which retains the biological activity is another option, but the best scenario would be to have a supply of the natural product that can be generated inexpensively and reproducibly in the lab under controlled conditions.Citation58
A Microbial Source for Manzamines
The isolation of manzamine alkaloids from a growing number of sponge genera indicates the possible existence of a sponge-associated microorganism as the actual biosynthetic source for the manzamine alkaloids.Citation2,Citation26-Citation28 This possibility was proposed by Kobayashi et al. in 1995Citation28 after six species of sponges were known to contain manzamines and appears even more likely now that an even greater diversity of sponges are known to yield manzamines. This possibility warrants careful investigation as the pharmaceutical potential of manzamine alkaloids continues to grow. If these compounds are actually produced by symbiotic bacteria within the sponge, isolation and culture of the producing bacteria may provide an efficient method for production of the compound in fermentation systems. This could ensure a steady supply of a particular manzamine in the high likelihood that one of these drug-leads will advance into clinical trials and applications. Certainly, if it could be shown that manzamines are microbial products, the pharmaceutical and biotechnology community would express a greater interest in this group of compounds and sponge metabolites in general.
Sustainable sourcing for development has been a strong justification for thorough microbiological evaluation of manzamine-producing sponges. In addition, evidence that sponge-associated microbes may play a significant role in the bioconversion of manzamines to the growing number of alkaloids found in manzamine producing sponges is provided by the biotransformation of 8-hydroxymanzamine A and its entantiomer to manzamine A and ent-12,34-oxamazamine F, respectively.Citation6,Citation38,Citation47
The culturable microbial communities associated with two Acanthostrongylophra species were investigated to obtain isolates that could be examined for manzamine production.Citation5,Citation6,Citation38,Citation47 A full molecular community analysis of the entire bacterial community (both culturable and unculturable) was completed for one of these species. This molecular approach is essential to explore the full diversity of microbes associated with sponges since typically less than 1% of the bacteria present are culturable by conventional approaches. Culturable isolates of heterotrophic bacteria were obtained from both species and unequivocally identified by 16S rRNA gene sequence analysis.
Diversity of Red Sea Biota
Our research groups at Suez Canal University (SCU) have been deeply involved in screening for production of biologically active compounds in microbes associated with marine macrobiota. We have isolated many different marine bacterial and fungal strains producing antimicrobial compounds and maintained the marine bacteria culture collection. A total of 453 marine bacterial isolates were obtained in pure culture from Red Sea sponges and cryopreserved. Based on colony morphology, 110 of these isolates were classified as putative actinomycetes. Both putative actinomycetes (grown on actinomycete selective media) and heterotrophic bacteria were screened for biologically active alkaloid production. Out of the 453 bacterial isolates tested, 95 isolates were positive for alkaloid production. A total of 25 of these isolates were putative actinomycetes. The culturable and unculturable microbial community associated with two manzamine-producing Red Sea Demosponges, Hyrtios erectus and Amphimedon sp were investigated and phylogenetic analysis revealed that the organisms isolated were from diverse phylogenetic groups, including Proteobacteria, Actinobacteria, Firmicutes, and Acidobacteria.Citation59-Citation61 Thirty five heterotrophic bacterial isolates, including actinomycetes and α-proteobacteria, were isolated in a preliminary effort to identify a possible microbial origin for these compounds. These isolates are currently under investigations for manzamine production. In addition, our work provides an excellent resource of several candidate bacteria for production of novel pharmaceutically important compounds.
Biosynthetic Potentials of Red Sea Biota
Recent studies have revealed that sponge-associated microbes are often the producers of biologically active secondary metabolitesCitation62,Citation63 and, as such, there is the potential for cultivation of the producing microorganism and isolation of the bioactive compound. However, if the producing microbe is unculturable, biotechnology can be used to isolate and clone the natural product’s biosynthetic genes and produce the compound in a heterologous host.Citation64 This has been accomplished for several compounds, including the polyketide antibiotic erythromycinCitation65,Citation66 and the cytotoxic polyketide-non-ribosomal peptide hybrids epothiloneCitation67-Citation69 and yersiniabactin.Citation70 The modular nature of polyketide synthases (PKSs) and non-ribosomal peptide synthetases (NRPSs) make these product biosynthetic enzymes amenable to cloning and heterologous production. NRPSs are multimodular enzymes in which each module is responsible for the addition of a single amino acid. These enzymes produce nonribosomal peptides (NRPs) by a thiotemplate mechanism. NRPSs can incorporate proteinogenic and non-protein amino acids, as well as carboxy and hydroxyl acids. Further modifications, such as N-methylation, epimerization, cyclization and heterocyclic ring formation, can also be performed by these enzymes.Citation71 NRPSs occur in a wide range of organisms, including bacteria, fungi, plants and marine organisms. These compounds often possess desirable pharmaceutical characteristics, and have thus been used for many applications, including use as siderophores, antibiotics, immunosuppressants and anticancer drugs (Dirk Konz 1999). So far, the majority of NRPSs and their products have been characterized in bacteria, especially Bacillus and Streptomyces species.
In a recent study,Citation72 12 PKS gene fragments were PCR amplified from Aspergillus sp isolated from Sharm El Sheik, Red Sea, Egypt using degenerative primer pairs. Sequence analysis reveals that the amplified PCR gene fragments show similarity to highly reduced (HR-PKS), six fragments, partially reduced (PR-PKSs) (also called methylsalicylic acid synthase (MSAS)-type, two fragments, and non-reducing (NR) PKSs (also called WA-type) PKS gene fragments in the database. Also, we report the characterization of a nonribosomal peptide synthetase (NRPS) gene in Aspergillus sp isolated from Sharm El Sheik, Egypt.
In addition, PKS gene fragments were PCR amplified from Negombata magnifica derived bacteria obtained from Red Sea (unpublished data). The KS domains were diverge from KSs from well-characterized clusters such as erythromycin,Citation73 pikromycin,Citation74 and epothilone,Citation75,Citation76 which is structurally very similar to latrunculin.
Future Directions
This review sheds the light on manzamines as unique and viable leads against infectious diseases, cancer, and inflammatory diseases. If local and international organizations provide better funding for manzmaine research, manzamines would give antimalarial drug discovery and vaccine development a decisive boosts and optimistically, signal the demise of a scourge that has plagued humankind with such impunity. The isolation of these alkaloids from a wide range of sponge genera has led to the speculation that these intriguing structures maybe of microbial origin. The Red Sea biodiversity, although represents an excellent source for drug discovery and development. The lack of funding requires international funding. In the absence of a legal formula to protect the right of Egypt as the country of origin, authorities will block the funding and the research. We strongly advocate expanding the exploration of Red Sea biota as an untapped source of novel microorganisms, which may serve as the leads and scaffolds for elaboration into desperately needed efficacious drugs for a multitude of diseases.
The diversity of alkaloid biosynthesis gene clusters hinders their exploration in microbial biota. In future, mining the Red Sea microbial genomes for biosynthetic gene clusters could booster the discovery of new classes of manzamine related compounds.
References
- Sakai R, Higa T, Jefford CW, Bernardinelli G. Manzamine A, a Novel Antitumor Alkaloid from a Sponge. J Am Chem Soc 1986; 108:6404 - 5; http://dx.doi.org/10.1021/ja00280a055
- Baldwin JE, Whitehead RC. On the biosynthesis of the manzamines. Tetrahedron Lett 1992; 33:2059 - 62; http://dx.doi.org/10.1016/0040-4039(92)88141-Q
- Ohtani II, Ichiba T, Isobe M, Kelly-Borges M, Scheuer PJ. Kauluamine, an unprecedented manzamine dimer from an Indonesian marine sponge, Prianos sp. J Am Chem Soc 1995; 117:10743 - 4; http://dx.doi.org/10.1021/ja00148a017
- El Sayed KA, Kelly M, Kara UAK, Ang KKH, Katsuyama I, Dunbar DC, et al. New manzamine alkaloids with potent activity against infectious diseases. J Am Chem Soc 2001; 123:1804 - 8; http://dx.doi.org/10.1021/ja002073o; PMID: 11456797
- Peng J, Hu J-F, Kazi AB, Li Z, Avery M, Peraud O, et al. Manadomanzamines A and B: a novel alkaloid ring system with potent activity against mycobacteria and HIV-1. J Am Chem Soc 2003; 125:13382 - 6; http://dx.doi.org/10.1021/ja030087z; PMID: 14583033
- Yousaf M, El Sayed KA, Rao KV, Lim CW, Hu JF, Kelly M, et al. 12,34-Oxamanzamines, novel biocatalytic and natural products from manzamine producing Indo-Pacific sponges. Tetrahedron 2002; 58:7397 - 402; http://dx.doi.org/10.1016/S0040-4020(02)00825-6
- Sakai R, Kohmoto S, Higa T, Jefford CW. Bernardinelli Gr. Manzamine B and C, two novel alkaloids from the sponge haliclona sp. Tetrahedron Lett 1987; 28:5493 - 6; http://dx.doi.org/10.1016/S0040-4039(00)96762-5
- Seki H, Nakagawa M, Hashimoto A, Hino T. The 1H- and 13C-Nuclear Magnetic Resonance Spectra of Manzamine C and Related Compounds. Chem Pharm Bull (Tokyo) 1993; 41:1173 - 6; http://dx.doi.org/10.1248/cpb.41.1173
- Koren-Goldshlager G, Kashman Y, Schleyer M. Haliclorensin, a Novel Diamino Alkaloid from the Marine Sponge Haliclonatulearensis. J Nat Prod 1998; 61:282 - 4; http://dx.doi.org/10.1021/np970442x; PMID: 9548861
- Guo Y, Trivellone E, Scognamiglio G, Cimino G. Misenine, a novel macrocyclic alkaloid with an unusual skeleton from the Mediterranean sponge Reniera sp. Tetrahedron 1998; 54:541 - 50; http://dx.doi.org/10.1016/S0040-4020(97)10314-3
- Cimino G, Scognamiglio G, Spinella A, Trivellone E. Structural Studies on Saraine A. J Nat Prod 1990; 53:1519 - 25; http://dx.doi.org/10.1021/np50072a019
- Ihara M, Fukumoto K. Recent progress in the chemistry of non-monoterpenoid indole alkaloids. Nat Prod Rep 1995; 12:277 - 301; http://dx.doi.org/10.1039/np9951200277
- Kobayashi J, Tsuda M, Kawasaki N, Sasaki T, Mikami Y. 6-Hydroxymanzamine A and 3,4-dihydromanzamine A, new alkaloids from the Okinawan marine sponge Amphimedon Sp. J Nat Prod 1994; 57:1737 - 40; http://dx.doi.org/10.1021/np50114a021; PMID: 7714542
- Tsuda M. Kobayashi Ji. Structures and Biogenesis of Manzamines and Related Alkaloids. Heterocycles 1997; 46:765 - 94; http://dx.doi.org/10.3987/REV-97-SR5
- Watanabe D, Tsuda M, Kobayashi J. Three new manzamine congeners from amphimedon sponge. J Nat Prod 1998; 61:689 - 92; http://dx.doi.org/10.1021/np970564p; PMID: 9599281
- Tsuda M, Watanabe D, Kobayashi JI. A New Manzamine Congener from Marine Sponge Amphimedon sp. 1999; 50:485-8.
- Tsuda M, Watanabe D. Kobayashi Ji. Ma'eganedin A, a new manzamine alkaloid from Amphimedon sponge. Tetrahedron Lett 1998; 39:1207 - 10; http://dx.doi.org/10.1016/S0040-4039(97)10842-5
- Kobayashi J, Tsuda M, Ishibashi M. Bioactive products from marine micro- and macro-organisms. Pure Appl Chem 1999; 71:1123 - 6; http://dx.doi.org/10.1351/pac199971061123
- Tsuda M, Kawasaki N. Kobayashi Ji. Keramaphidin C and keramamine C plausible biogenetic precursors of manzamine C from an Okinawan marine sponge. Tetrahedron Lett 1994; 35:4387 - 8; http://dx.doi.org/10.1016/S0040-4039(00)73363-6
- Tsuda M, Kawasaki N. Kobayashi Ji. Ircinols A and B, first antipodes of manzamine-related alkaloids from an Okinawan marine sponge. Tetrahedron 1994; 50:7957 - 60; http://dx.doi.org/10.1016/S0040-4020(01)85280-7
- Ji K. Tsuda M, Kawasaki N, Matsumoto K, Adachi T. Keramaphidin B, a novel pentacyclic alkaloid from a marine sponge Amphimedon sp.: A plausible biogenetic precursor of manzamine alkaloids. Tetrahedron Lett 1994; 35:4383 - 6; http://dx.doi.org/10.1016/S0040-4039(00)73362-4
- Ji K, Kawasaki N, Tsuda M. Absolute stereochemistry of keramaphidin B. Tetrahedron Lett 1996; 37:8203 - 4; http://dx.doi.org/10.1016/0040-4039(96)01861-8
- Tsuda M, Inaba K, Kawasaki N, Honma K. Kobayashi Ji. Chiral resolution of (±)-keramaphidin B and isolation of manzamine L, a new Î2-carboline alkaloid from a sponge Amphimedon sp. Tetrahedron 1996; 52:2319 - 24; http://dx.doi.org/10.1016/0040-4020(95)01057-2
- Ji K. Watanabe D, Kawasaki N, Tsuda M. Nakadomarin A, a Novel Hexacyclic Manzamine-Related Alkaloid from Amphimedon Sponge. J Org Chem 1997; 62:9236 - 9; http://dx.doi.org/10.1021/jo9715377
- Crews P, Cheng X-C, Adamczeski M. RodrÃguez J, Jaspars M, Schmitz FJ, Traeger SC, Pordesimo EO. 1,2,3,4-tetrahydro-8-hydroxymanzamines, alkaloids from two different haplosclerid sponges. Tetrahedron 1994; 50:13567 - 74; http://dx.doi.org/10.1016/S0040-4020(01)85671-4
- Ichiba T, Sakai R, Kohmoto S, Saucy G. New manzamine alkaloids from a sponge of the genus xestospongia. Tetrahedron Lett 1988; 29:3083 - 6; http://dx.doi.org/10.1016/0040-4039(88)85091-3
- Kitagawa I, Kobayashi M. Antitumor Marine Natural Products. Yuki Gosei Kagaku Kyokaishi 1991; 49:1053 - 61; http://dx.doi.org/10.5059/yukigoseikyokaishi.49.1053
- Kobayashi M, Chena Y-J, Aokia S, Inb Y, Ishidab T, Kitagawaa I. Four new β-carboline alkaloids isolated from two Okinawan marine sponges of Xestospongia sp. and Haliclona sp. Tetrahedron 1995; 51:3727 - 36; http://dx.doi.org/10.1016/0040-4020(95)95723-9
- Rodriguez J, Crews P. Revised structure of xestocyclamine A and description of a new analogue. Tetrahedron Lett 1994; 35:4719 - 22; http://dx.doi.org/10.1016/S0040-4039(00)76950-4
- Rodriguez J, Peters BM, Kurz L, Schatzman RC, McCarley D, Lou L, et al. An alkaloid protein kinase C inhibitor, xestocyclamine A, from the marine sponge Xestospongia sp. J Am Chem Soc 1993; 115:10436 - 7; http://dx.doi.org/10.1021/ja00075a100
- Ohtani II, Ichiba T, Isobe M, Kelly-Borges M, Scheuer PJ. Kauluamine, an unprecedented manzamine dimer from an Indonesian marine sponge, Prianos sp. J Am Chem Soc 1995; 117:10743 - 4; http://dx.doi.org/10.1021/ja00148a017
- Edrada RA, Proksch P, Wray V, Witte L, Müller WE, Van Soest RW. Four new bioactive manzamine-type alkaloids from the Philippine marine sponge Xestospongia ashmorica. J Nat Prod 1996; 59:1056 - 60; http://dx.doi.org/10.1021/np9604083; PMID: 8946747
- Kong F, Andersen RJ, Allen TM. Ingamines A and B, new cytotoxic alkaloids from the marine sponge Xestospongia ingens. Tetrahedron 1994; 50:6137 - 44; http://dx.doi.org/10.1016/S0040-4020(01)80635-9
- Kong F, Andersen RJ. Ingenamine alkaloids isolated from the sponge Xestospongia ingens: Structures and absolute configurations. Tetrahedron 1995; 51:2895 - 906; http://dx.doi.org/10.1016/0040-4020(95)00043-8
- Kong F, Andersen RJ, Allen TM. Ingenamine, a novel pentacyclic alkaloid from the marine sponge Xestospongia ingens. Tetrahedron Lett 1994; 35:1643 - 6; http://dx.doi.org/10.1016/0040-4039(94)88308-4
- Kong F, Andersen RJ, Allen TM. Madangamine A, a novel cytotoxic alkaloid from the marine sponge Xestospongia ingens. J Am Chem Soc 1994; 116:6007 - 8; http://dx.doi.org/10.1021/ja00092a077
- Kong F, Graziani EI, Andersen RJ. Madangamines B-E, Pentacyclic Alkaloids from the Marine Sponge Xestospongia ingens.. J Nat Prod 1998; 61:267 - 71; http://dx.doi.org/10.1021/np970377r; PMID: 9548859
- Kasanah N, Yousaf M, Rao KV, Wedge D, Hamann MT. The biocatalytic conversion of 8-hydroxymanzamine A to manzamine A using Fusarium solani. Tetrahedron Lett 2002; 44:1291 - 3; http://dx.doi.org/10.1016/S0040-4039(02)02816-2
- Rao KV, Santarsiero BD, Mesecar AD, Schinazi RF, Tekwani BL, Hamann MT. New manzamine alkaloids with activity against infectious and tropical parasitic diseases from an Indonesian sponge. J Nat Prod 2003; 66:823 - 8; http://dx.doi.org/10.1021/np020592u; PMID: 12828469
- Ichiba T, Corgiat JM, Scheuer PJ, Kelly-Borges M. 8-Hydroxymanzamine A, a beta-carboline alkaloid from a sponge, Pachypellina sp. J Nat Prod 1994; 57:168 - 70; http://dx.doi.org/10.1021/np50103a027; PMID: 8158160
- Nakamura H, Deng S, Kobayashi J, Ohizumi Y, Tomotake Y, Matsuzaki T, et al. Physiologically active marine natural products from Porifera. XV. Keramamine-A and -B, novel antimicrobial alkaloids from the Okinawan marine sponge Pellina sp. Tetrahedron Lett 1987; 28:621 - 4; http://dx.doi.org/10.1016/S0040-4039(00)95796-4
- Williams DE, Lassota P, Andersen RJ. Motuporamines A to C, Cytotoxic Alkaloids Isolated from the Marine Sponge Xestospongia exigua (Kirkpatrick). J Org Chem 1998; 63:4838 - 41; http://dx.doi.org/10.1021/jo980355p
- Bourguet-Kondracki ML, Martin MT, Guyot M. A new β-carboline alkaloid isolated from the marine sponge Hyrtios erecta. Tetrahedron Lett 1996; 37:3457 - 60; http://dx.doi.org/10.1016/0040-4039(96)00573-4
- Kondo K, Shigemori H, Kikuchi Y, Ishibashi M, Sasaki T, Kobayashi J. Ircinals A and B from the Okinawan Marine Sponge, Ircinia sp.: Plaisible Biogenetic Precoursors of Manzamine Alkaloids. Org Chem 1992; 57:2480 - 3; http://dx.doi.org/10.1021/jo00034a052
- Zhou B-N, Slebodnick C, Johnson RK, Mattern MR, Kingston DGI. New Cytotoxic Manzamine Alkaloids from a Palaun Sponge. Tetrahedron 2000; 56:5781 - 4; http://dx.doi.org/10.1016/S0040-4020(00)00534-2
- Williams DE, Craig KS, Patrick B, McHardy LM, van Soest R, Roberge M, et al. Motuporamines, anti-invasion and anti-angiogenic alkaloids from the marine sponge Xestospongia exigua (Kirkpatrick): isolation, structure elucidation, analogue synthesis, and conformational analysis. J Org Chem 2002; 67:245 - 58; http://dx.doi.org/10.1021/jo016101c; PMID: 11777468
- Kasanah N, Rao K, Yousaf M, Wedge DE, Hill RT, Hamann MT. Biotransformation studies of the manzamine alkaloids. Mar Biotechnol (NY) 2004; 6:268 - 72
- Higa T, Sakai R, Kohmoto S, Lui MS. Isolation of Antitumor Manzamines B, C, and D from Haliclona. In: application Ep, ed., 1988:44.
- Toshima K, Ohta K, Ohashi A, Nakamura T, Nakata M, Tatsuta K, et al. Molecular Design, Chemical Synthesis, and Study of Novel Enediyne-Sulfide Systems Related to the Neocarzinostatin Chromophore. J Am Chem Soc 1995; 117:4822 - 31; http://dx.doi.org/10.1021/ja00122a012
- El Sayed KA, Dunbar DC, Perry TL, Wilkins SP, Hamann MT, Greenplate JT, et al. Marine Natural Products as Prototype Insecticidal Agents. J Agric Food Chem 1997; 45:2735 - 9; http://dx.doi.org/10.1021/jf960746+
- Mayer AMS, Gunasekera SP, Pomponi SA, Sennett SH. Anti-inflammatory uses of manzamine. US 2002.
- Yousaf M, Hammond NL, Peng J, Wahyuono S, McIntosh KA, Charman WN, et al. New manzamine alkaloids from an Indo-Pacific sponge. Pharmacokinetics, oral availability, and the significant activity of several manzamines against HIV-I, AIDS opportunistic infections, and inflammatory diseases. J Med Chem 2004; 47:3512 - 7; http://dx.doi.org/10.1021/jm030475b; PMID: 15214779
- Pc H, Frank P, Roggo S, Hamann MT. PCT International Patent Application. 2006:24.
- Peng J, Rao KV, Choo YM, Hamann MT. Manzamine Alkaloids. Modern Alkaloids. Wiley-VCH Verlag GmbH & Co. KGaA. 2007:189-232.
- Winkler JD, Axten JM. The First Total Syntheses of Ircinol A, Ircinal A, and Manzamines A and D. J Am Chem Soc 1998; 120:6425 - 6; http://dx.doi.org/10.1021/ja981303k
- Peng J, Kudrimoti S, Prasanna S, Odde S, Doerksen RJ, Pennaka HK, et al. Structure-activity relationship and mechanism of action studies of manzamine analogues for the control of neuroinflammation and cerebral infections. J Med Chem 2010; 53:61 - 76; http://dx.doi.org/10.1021/jm900672t; PMID: 20017491
- Magnier E. Langlois Yv. Manzamine Alkaloids, Syntheses and Synthetic Approaches. Tetrahedron 1998; 54:6201 - 58; http://dx.doi.org/10.1016/S0040-4020(98)00357-3
- Hildebrand M, Waggoner LE, Lim GE, Sharp KH, Ridley CP, Haygood MG. Approaches to identify, clone, and express symbiont bioactive metabolite genes. Nat Prod Rep 2004; 21:122 - 42; http://dx.doi.org/10.1039/b302336m; PMID: 15039839
- Abdelmohsen UR, Pimentel-Elardo SM, Hanora A, Radwan M, Abou-El-Ela SH, Ahmed S, et al. Isolation, phylogenetic analysis and anti-infective activity screening of marine sponge-associated actinomycetes. Mar Drugs 2010; 8:399 - 412; http://dx.doi.org/10.3390/md8030399; PMID: 20411105
- Radwan M, Hanora A, Abo-Elmatty DM, Abou-El-Ela SH. Diversity of culturable actinobacteria isolated from two Red Sea sponges. Suez Canal Univ Med J 2009; 23
- Radwan M, Hanora A, Zan J, Mohamed NM, Abo-Elmatty DM, Abou-El-Ela SH, et al. Bacterial community analyses of two Red Sea sponges. Mar Biotechnol (NY) 2010; 12:350 - 60; http://dx.doi.org/10.1007/s10126-009-9239-5; PMID: 19957096
- Hentschel U, Usher KM, Taylor MW. Marine sponges as microbial fermenters. FEMS Microbiol Ecol 2006; 55:167 - 77; http://dx.doi.org/10.1111/j.1574-6941.2005.00046.x; PMID: 16420625
- Piel J. Metabolites from symbiotic bacteria. Nat Prod Rep 2009; 26:338 - 62; http://dx.doi.org/10.1039/b703499g; PMID: 19240945
- Fujii I. Heterologous expression systems for polyketide synthases. Nat Prod Rep 2009; 26:155 - 69; http://dx.doi.org/10.1039/b817092b; PMID: 19177221
- Kao CM, Katz L, Khosla C. Engineered biosynthesis of a complete macrolactone in a heterologous host. Science 1994; 265:509 - 12; http://dx.doi.org/10.1126/science.8036492; PMID: 8036492
- Wang Y, Boghigian BA, Pfeifer BA. Improving heterologous polyketide production in Escherichia coli by overexpression of an S-adenosylmethionine synthetase gene. Appl Microbiol Biotechnol 2007; 77:367 - 73; http://dx.doi.org/10.1007/s00253-007-1172-9; PMID: 17876579
- Julien B, Shah S. Heterologous expression of epothilone biosynthetic genes in Myxococcus xanthus.. Antimicrob Agents Chemother 2002; 46:2772 - 8; http://dx.doi.org/10.1128/AAC.46.9.2772-2778.2002; PMID: 12183227
- Mutka SC, Carney JR, Liu YQ, Kennedy J. Heterologous production of epothilone C and D in Escherichia coli.. Biochemistry 2006; 45:1321 - 30; http://dx.doi.org/10.1021/bi052075r; PMID: 16430229
- Tang L, Shah S, Chung L, Carney J, Katz L, Khosla C, et al. Cloning and heterologous expression of the epothilone gene cluster. Science 2000; 287:640 - 2; http://dx.doi.org/10.1126/science.287.5453.640; PMID: 10649995
- Pfeifer BA, Wang CCC, Walsh CT, Khosla C. Biosynthesis of Yersiniabactin, a complex polyketide-nonribosomal peptide, using Escherichia coli as a heterologous host. Appl Environ Microbiol 2003; 69:6698 - 702; http://dx.doi.org/10.1128/AEM.69.11.6698-6702.2003; PMID: 14602630
- Jirakkakul J, Punya J, Pongpattanakitshote S, Paungmoung P, Vorapreeda N, Tachaleat A, et al. Identification of the nonribosomal peptide synthetase gene responsible for bassianolide synthesis in wood-decaying fungus Xylaria sp. BCC1067. Microbiology 2008; 154:995 - 1006; http://dx.doi.org/10.1099/mic.0.2007/013995-0; PMID: 18375793
- Hanora A, Crow S. Exploring the Potential of Biosynthetic Gene Clusters in Sponge Associated Fungi from Red Sea, Egypt. New Egypt J Microbiol 2010; 27:182 - 98
- Donadio S, Staver MJ, McAlpine JB, Swanson SJ, Katz L. Modular organization of genes required for complex polyketide biosynthesis. Science 1991; 252:675 - 9; http://dx.doi.org/10.1126/science.2024119; PMID: 2024119
- Xue YQ, Zhao LS, Liu HW, Sherman DH. A gene cluster for macrolide antibiotic biosynthesis in Streptomyces venezuelae: architecture of metabolic diversity. Proc Natl Acad Sci USA 1998; 95:12111 - 6; http://dx.doi.org/10.1073/pnas.95.21.12111; PMID: 9770448
- Julien B, Shah S, Ziermann R, Goldman R, Katz L, Khosla C. Isolation and characterization of the epothilone biosynthetic gene cluster from Sorangium cellulosum. Gene 2000; 249:153 - 60; http://dx.doi.org/10.1016/S0378-1119(00)00149-9; PMID: 10831849
- Molnár I, Schupp T, Ono M, Zirkle RE, Milnamow M, Nowak-Thompson B, et al. The biosynthetic gene cluster for the microtubule-stabilizing agents epothilones A and B from Sorangium cellulosum So ce90. Chem Biol 2000; 7:97 - 109; http://dx.doi.org/10.1016/S1074-5521(00)00075-2; PMID: 10662695