Abstract
Checkpoint kinase 1 (Chk1) plays key roles in all currently defined cell cycle checkpoints, but its functions in mouse oocyte meiosis remain unclear. In this study, we report the expression, localization and functions of Chk1 in mouse oocyte meiosis. Chk1 was expressed from germinal vesicle (GV) to metaphase II (MII) stages and localized to the spindle from pro-metaphase I (pro-MI) to MII stages in mouse oocytes. Chk1 depletion facilitated the G2/M transition while Chk1 overexpression inhibited the G2/M transition as indicated by germinal vesicle breakdown (GVBD), through regulation of Cdh1 and Cyclin B1. Chk1 depletion did not affect meiotic cell cycle progression after GVBD, but its overexpression after GVBD activated the spindle assembly checkpoint and prevented homologous chromosome segregation, thus arresting oocytes at pro-MI or metaphase I (MI) stages. These results suggest that Chk1 is indispensable for prophase I arrest and functions in G2/M checkpoint regulation in meiotic oocytes. Moreover, Chk1 overexpression affects meiotic spindle assembly checkpoint regulation and thus chromosome segregation.
Introduction
Mammalian oocytes are arrested at prophase I from birth until puberty. Prophase I arrest and progression from metaphase I (MI) to metaphase II (MII) are two pivotal phases during oocyte meiotic maturation in mammals.Citation1 Resumption of meiosis, which is morphologically characterized by germinal vesicle breakdown (GVBD), is closely associated with regulation of cyclin-dependent kinase 1 (CDK1) activity.Citation2 In prophase I-arrested oocytes, CDK1 activity is maintained at a low level by several factors. Cyclin B1, a protein associated with CDK1, activating CDK1 by altering its phosphorylation status, is degraded by the anaphase-promoting complex-Cdh1 (APC-Cdh1) in prophase I-arrested oocytes.Citation2-Citation4 Wee1/Myt kinase family negatively regulates CDK1 by phosphorylating CDK1 on its Thr14 and Tyr15 sites. Cdc25 phosphatases, including Cdc25A, Cdc25B and Cdc25C in mammals, are responsible for the dephosphorylation of these sites on CDK1.Citation5,Citation6 The equilibrium between the kinase activities of Wee1/Myt kinase family and the phosphatase activities of Cdc25 phosphatases determines whether the oocytes resume meiosis through regulating CDK1 activity.Citation3 Many other proteins, such as phosphodiesterase 3A (PDE3A), protein kinase A, protein kinase C, Aurora kinase A, Polo-like kinase 1 (Plk1) and BubR1 are also involved in the resumption of meiosis in mammalian oocytes.Citation1,Citation3,Citation7,Citation8
The DNA damage transducer checkpoint kinase 1 (Chk1) is involved in all currently defined cell cycle checkpoints.Citation9,Citation10 In the G2/M checkpoint, Cdc25A phosphorylation by Chk1 leads to the ubiquitination and proteasomal degradation of Cdc25A.Citation9,Citation11 Chk1 also phosphorylates Wee1, rendering it more stable.Citation9 Moreover, Chk1-/- mice showed aberrant cell cycle checkpoint function and early embryonic death, and the G2/M checkpoint is abrogated in Chk1-/- embryo cells.Citation12
Chk1 also functions in the spindle assembly checkpoint (SAC) regulation. The SAC protects against chromosome missegregation by delaying sister chromatid separation until all sister kinetochores have achieved bipolar attachment to spindle microtubules in mitosis.Citation13 During meiotic maturation of mouse oocytes, SAC proteins such as mitotic arrest-deficient-1 (Mad1), Mad2, budding uninhibited by benzimidazole-1 (Bub1), Bub3, BubR1 and monopolar spindle 1 (Msp1) also play important roles.Citation1,Citation14-Citation23 Chk1-depleted cells display metaphase block, chromosome misalignment in metaphase, chromosome lagging in anaphase and kinetochore defects, which are caused by negative regulation of Plk1 by Chk1.Citation24 Another study reports different results showing that Chk1 is required for the spindle assembly checkpoint by phosphorylating Aurora B and mediating phosphorylation and kinetochore localization of BubR1.Citation25 Moreover, in vivo studies show that Chk1+/- mouse cells exhibit multiple mitotic defects, increased binucleation and mislocalization of Aurora B during mitosis.Citation26 Thus, Chk1 is important for both G2/M checkpoint and completion of mitosis. In addition, Chk1 was reported to be involved in meiotic resumption in Xenopus oocytes.Citation27 Chk1 and Chk2 functions are needed for the transition of female germ cells to exit the mitotic cell cycle and enter meiosis.Citation28
We hypothesized that Chk1 may play a role during mouse oocyte meiotic maturation. Using RNA interference (RNAi) and overexpression approaches, we have shown that Chk1 is indispensable for the prophase I arrest of mouse oocytes. Furthermore, overexpression of Chk1 arrests oocytes at the Pro-MI/MI stage and prevents homologous chromosome segregation.
Results
Expression and localization of Chk1 during mouse oocyte meiotic maturation.
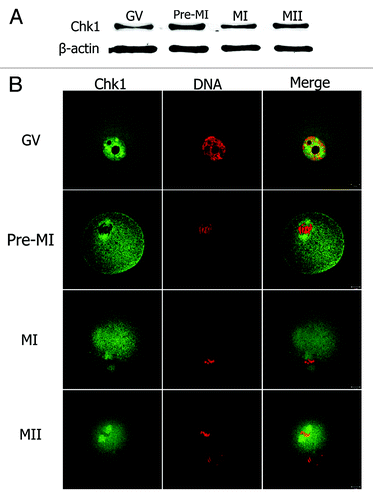
To study the functions of Chk1 during mouse oocyte maturation, we first examined the expression and subcellular localization of Chk1. Samples were collected after oocytes had been cultured for 0, 4, 8 and 12 h, corresponding to GV, Pro-MI, MI and MII stages, respectively. Western blot analysis showed that Chk1 was expressed at every stage of oocyte maturation (). To examine the subcellular distribution of Chk1, immunofluorescent staining was performed on oocytes at different stages. Chk1 localized in the nucleus at the GV stage (). At Pro-MI, MI and MII stages, Chk1 mainly was localized to the spindle (). To confirm the specificity of the Chk1 antibody, we injected myc6-Chk1 mRNA into mouse oocytes and stained the oocytes with anti-myc antibody. The results showed that myc-Chk1 localized in the germinal vesicle at the GV stage and localized to the spindle at the MI stage (Fig. S1), which supported our data obtained with anti-Chk1 antibody staining.
Figure 1. Expression and subcellular localization of Chk1. (A) Oocyte samples were collected for western blot after 0 h, 4 h, 8 h or 12 h of culture, corresponding to GV, Pro-MI, MI and MII stages, respectively. The molecular weight of Chk1 is 56 kDa and β-tubulin is 42 kDa. (B) Oocytes at GV, pro-MI, MI or MII stages were co-stained with anti-Chk1 antibody (green) and PI (red), and the slides were examined under a confocal microscope. Bar, 10 μm.
Depletion of Chk1 facilitated GVBD by regulating expression of Cdh1 and cyclin B1.
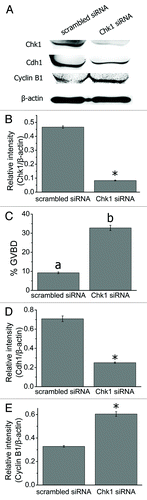
We first injected siRNAs into mouse oocytes at the GV stage to study the roles of Chk1 during meiosis. Injected oocytes were arrested at the GV stage in milrinone-containing (2.5 M) M2 medium for 24 h and collected for western blot analysis. Compared with the scrambled siRNA injection group, the expression level of Chk1 was significantly reduced in the Chk1 siRNA-injected group (0.08 ± 0.003) compared with that in the control group (0.47 ± 0.008, p < 0.05, ). Next, we arrested siRNA-injected oocytes in M2 medium containing 0.75 M milrinone (which was the minimum concentration of milrinone to maintain meiotic arrest in mouse oocytesCitation29) for 24 h and counted the number of GVBD oocytes. The percentage of GVBD oocytes in the Chk1 siRNA injection group (32.78 ± 1.35%, n = 166) was considerably higher than that in the scrambled siRNA injection group (9.27 ± 0.47%, n = 152, p < 0.001, ). We tested Cdc25A in Chk1-depleted oocytes. The level of Cdc25A increased slightly in the Chk1 depletion group (0.84 ± 0.015) compared with that in the control group (0.59 ± 0.02, p < 0.05, Fig. S2A and B). We next examined the expression of Cdh1 in Chk1-depleted oocytes and found that it was remarkably reduced (0.71 ± 0.03 in the control group and 0.25 ± 0.007 in the Chk1-depleted group, p < 0.05, ). We then tested the expression of cyclin B1 in Chk1 siRNA-injected oocytes. The level of cyclin B1 in the Chk1-depleted group (0.60 ± 0.02) was significantly higher than that in the control group (0.31 ± 0.007, p < 0.05, ).
Figure 2. Chk1 depletion facilitated GVBD in mouse oocytes. (A) Western blot for Chk1, Cdh1 (55 kDa) and Cyclin B1 (60 kDa) (n = 200 oocytes each lane). GV stage oocytes were injected with scrambled or Chk1 siRNA and arrested in M2 medium containing 2.5 μM milrinone for 24 h before being collected for western blot. (B) Gray-scale analysis of Chk1. Volume analysis in the software Quantity One (Bio-Rad) was used to evaluate the relative levels of Chk1 after Chk1 siRNA injection. Levels of expression were normalized to levels of β-actin and each bar represents mean ± SEM (n = 3). *, p < 0.05. (C) The rates of GVBD oocytes in the scrambled and Chk1 siRNA injected group after being arrested for 24 h in M2 medium containing 0.75 μM milrione after injection. Data are presented as mean ± SE. The superscripts a, b on top of the bars represent ratios of GVBD oocytes that differ significantly between the two groups (p < 0.001). (D-E) Gray-scale analysis of Cdh1 and Cyclin B1. The relative levels of Cdh1 and Cyclin B1 after Chk1 siRNA injection were evaluated by gray-scale analysis using the software Quantity One (Bio-Rad). Levels of expression were normalized to levels of β-actin and each bar represents mean ± SEM (n = 3). *, p < 0.05.
Overexpression of Chk1 inhibited oocyte meiotic resumption by downregulating cyclin B1.
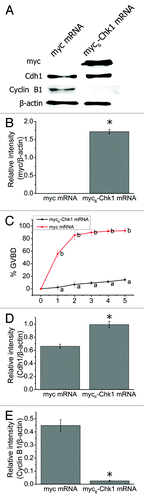
We injected myc6-Chk1 mRNA into mouse oocytes in the GV stage. The oocytes were arrested in M2 medium containing 2.5 M milrinone for 2 h to allow expression of the protein before collecting the oocytes for western blot analysis. The molecular weight of myc tag and Chk1 is 10 kD and 56 kD, respectively. Using myc antibody, we detected a band about 66 kD in the injection group, indicating that exogenous myc6-Chk1 was successfully expressed in mouse oocytes (1.72 ± 0.06 in the Chk1 overexpressed group compared with 0.00 in the control group, p < 0.05, ). We counted the number of GVBD oocytes at different time points after the oocytes were washed and transferred to milrinone-free M2 medium. In myc mRNA-injected oocytes, the rate of GVBD was 55.83% at 1 h; it rose to 85.36% at 2 h and remained stable after that (n = 166) (). In sharp contrast to these results, the rate of GVBD oocytes in the Chk1-overexpression group was much lower, with only 14.51% at 5 h of culture (n = 176) (). The rates of GVBD oocytes between the two groups differed significantly at the five time points examined (p < 0.001). As in the Chk1-depletion experiments, we examined Cdc25A, Cdh1 and cyclin B1 in Chk1-overexpressed oocytes. Cdc25A was reduced significantly in the Chk1-overexpression group (0.23 ± 0.05) compared with that in the control group (0.71 ± 0.04, p < 0.05, Fig. S2). Higher levels of Cdh1 were detected in the Chk1-overexpression group (0.99 ± 0.05) compared with that of the control group (0.66 ± 0.03, p < 0.05, ). In turn, the level of cyclin B1 in the Chk1-overexpression group (0.03 ± 0.004) was notably reduced compared with that in the control group (0.44 ± 0.04, p < 0.05, ).
Figure 3. Chk1 overexpression inhibited GVBD in mouse oocytes. (A) Western blot for Chk1, Cdh1 and Cyclin B1 (n = 200 oocytes each lane). GV stage oocytes were injected with myc or myc6-Chk1 mRNA and arrested in M2 medium containing 2.5 μM milrinone for 2 h before being collected for western blot. (B) Change of myc was calculated by gray-scale analysis using the software Quantity One (Bio-Rad). Levels of expression were normalized to levels of β-actin and each bar represents mean ± SEM (n = 3). *, p < 0.05. (C) The rates of GVBD oocytes in the myc or myc6-Chk1 mRNA injected group. The oocytes were washed thoroughly and cultured in milrinone-free M2 medium after 2 h arrest in 2.5 μM milrinone. Data are presented as mean percentage (mean ± SEM) of at least three independent experiments. Different superscripts indicate statistical difference (p < 0.001). (D-E) Gray-scale analysis of Cdh1 and Cyclin B1. The software Quantity One (Bio-Rad) was used to analyze the relative levels of Chk1, Cdh1 and Cyclin B1 after Chk1 mRNA injection. Levels of expression were normalized to levels of β-actin and each bar represents mean ± SEM (n = 3). *, p < 0.05.
Chk1 depletion did not affect oocyte meiotic progression after GVBD.
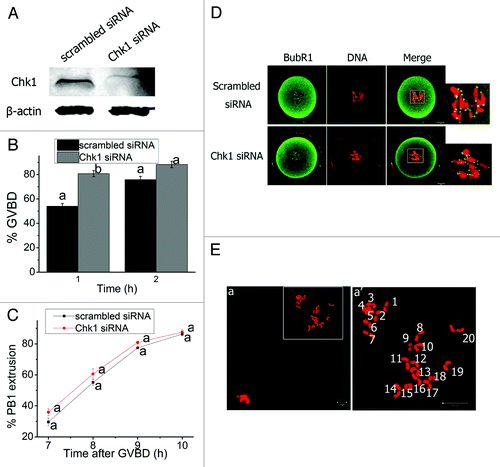
We studied the functions of Chk1 during meiosis by injecting specific siRNA into mouse oocytes, which efficiently knocked down Chk1 (). The percentage of GVBD oocytes in the Chk1-depletion group (75.82 ± 2.62%) was remarkably higher than that in the control group (54.06 ± 2.18%) at 1 h after release of the oocytes into milrinone-free M2 medium (p < 0.01) (). The GVBD rates were similar in the two groups at 2 h of culture (p > 0.05) (). This result indicates that Chk1 depletion was able to accelerate GVBD. However, Chk1 depletion had no effect on the timing of first polar body emission (p > 0.05) (), and the SAC protein BubR1 showed similar distribution patterns in oocytes from both groups at 4 h of culture (). Chromosome spread was performed to study the impact of Chk1 depletion on chromosome segregation. Chk1-depleted MII eggs displayed normal chromosome morphology and number (), implying that Chk1 depletion did not impair proper homologous chromosome segregation.
Figure 4. Chk1 depletion did not affect meiotic progression after GVBD in mouse oocytes. (A) Western blot analysis of the efficiency of Chk1 siRNA. Oocytes were injected with siRNAs and collected after being arrested for 24 h in M2 medium containing 2.5 μM milrinone. (B) Rates of GVBD oocytes in the scrambled siRNA or Chk1 siRNA injected oocytes. Oocytes were washed and cultured in milrinone-free M2 medium after being arrested for 24 h following siRNAs injection. Data are presented as mean percentage (mean ± SEM) of at least three independent experiments. Different superscripts indicate statistical difference (p < 0.01). (C) Timing of PB1 extrusion in scrambled and Chk1 siRNA injected oocytes. The superscript a next to the symbols representing the rates of PB1 extrusion between the two groups were not significantly different (p > 0.05). (D) Representative images of localization of BubR1 in scrambled and Chk1 siRNA injected groups. Oocytes arrested for 24 h in M2 medium containing milrinone following injection were washed and cultured in milrinone-free M2 medium for 4 h (Pro-MI) and co-stained with BubR1 (green) and PI (red). (E) Chromosome spread of Chk1-depleted oocytes in MII stage. The chromosome number was normal. a’ represents enlargement of the rectangle in a. Bars (D and E), 10 μm.
Chk1 overexpression activates SAC and arrests oocytes at pro-MI or MI stage.
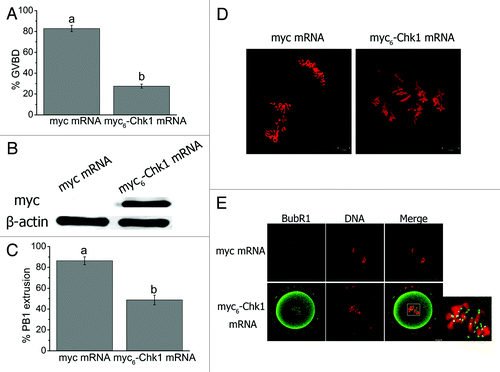
To study how Chk1 overexpression affects mouse oocyte meiotic maturation, the oocytes were collected in milrinone-free M2 medium and injected with Chk1 mRNA. After injection, the oocytes were cultured in milrinone-free M2 medium. At 2 h after culture, the GVBD rate in the Chk1-overexpressed group (27.55 ± 1.94%, n = 164) was significantly lower than that in the control group (82.84 ± 3.02%, n = 156, p < 0.001) (), indicating Chk1 mRNA may be translated before GVBD occurred. So we injected Chk1 mRNA into GVBD oocytes and found that the myc6-Chk1 was expressed in mouse oocytes (). After culture for 10 h, the PB1 extrusion rate in the Chk1 overexpressed group (48.73 ± 4.60%, n = 84) was remarkably lower than that in the control group (86.40 ± 3.79%, n = 98, p < 0.01, ). Chromosome spread showed that all chromosomes of the Chk1-overexpressed oocytes without PB1 (51.27 ± 4.60%) were in the bivalent state (). In contrast, chromosomes of most oocytes (86.40 ± 3.79%) in the control group were in the univalent state (). We fixed oocytes 8 h after mRNA injection and stained the oocytes with anti-BubR1 antibody and propidium iodide (PI). The chromosomes in the control oocytes were divided into two parts, and no signal of BubR1 was detected (). On the contrary, the chromosomes in the Chk1-overexpressed oocytes stayed as an aggregate and showed specific staining of BubR1 (), indicating SAC activation.
Figure 5. Chk1 overexpression arrested oocytes at MI or earlier stages, prevented homologous chromosome segregation and activated the spindle assembly checkpoint. (A) Rate of GVBD oocytes in myc and myc6-Chk1 mRNA injected groups at 2 h of injection. The oocytes were collected and cultured in milrinone-free M2 medium. Data are presented as mean percentage (mean ± SEM) of at least three independent experiments. Different superscripts indicate statistical difference (p < 0.001). (B) western blot analysis of myc tagged Chk1 expression. GVBD oocytes were injected in this test. (C) Percentage of PB1 extrusion in myc and myc6-Chk1 mRNA injected groups 10 h of injection. Data are presented as mean percentage (mean ± SEM) of at least three independent experiments. The superscripts a, b indicate statistical difference (p < 0.01). (D) Representative images of chromosome spreads of the oocytes in myc and myc6-Chk1 mRNA injected groups. (E) Detection of BubR1 in myc and myc6-Chk1 mRNA injected oocytes. Bars (D and E), 10 μm.
Discussion
In this study, we show that Chk1 is essential for GV stage arrest in mouse oocytes, which involves the regulation of Cdh1 and cyclin B1. Furthermore, overexpression of Chk1 prevents homologous chromosome segregation and first polar body extrusion, arresting mouse oocytes at the pro-MI or MI stage. To our knowledge, this is the first study on the role of Chk1 during mouse oocyte meiotic maturation.
Chk1 was reported to be expressed at every stage in the cell cycle of H1299 cells.Citation30 Here, we show in mouse oocytes that Chk1 is expressed from GV to MII stages. These data indicate that Chk1 may play a role not only in mitosis, but also in meiosis. The subcellular localization of Chk1 was controversial, with centrosomes, nuclear, cytoplasm and kinetochores being considered as possible sites.Citation25,Citation31-Citation33 We find that Chk1 is localized in the germinal vesicle and to the spindle from pro-MI to MII stages, and this is confirmed by myc6-tagged Chk1 staining. This localization pattern suggests that Chk1 may have different functions in mouse oocytes.
Chk1 acts as a transducer downstream of ATR [ATM (ataxia telangiectesia mutated) and Rad3 related] in the DNA damage checkpoints.Citation11,Citation34 Checkpoint kinases (Chk1 or Chk2) phosphorylate Cdc25 proteins, inactivating and keeping Cdc25 proteins out of the nucleus or by causing proteolytic degradation.Citation11,Citation35 This causes G1/S or G2/M arrest in human cell lines, because unphosphorylated Cdc25 proteins dephosphorylate Cdk2 and Cdc2, and the dephosphorylation of Cdk2 and Cdc2 promotes cell cycle progression.Citation31,Citation35,Citation36 Herein, we find that Chk1 functions in a similar way as in human cells. Chk1 depletion causes upregulation of Cdc25A and resumption of meiosis in mouse oocytes, while overexpression of Chk1 induces downregulation of Cdc25A and prevents GVBD in oocytes. The Chk1 siRNA cannot properly function in all oocytes, and the exogenous protein (myc6-Chk1) may fail to be expressed in a considerable number of oocytes. Considering this, the difference of GVBD rates between control and injected groups should be more significant. Furthermore, we show that the change in Chk1 level influences the expression level of Cdh1 and cyclin B1, which gives us new insights into the role of Chk1 in the G2/M checkpoint. The feedback control of Cdh1 by active Cdk1 may contribute to the level changes of Cdh1 in the Chk1-depleted or -overexpressed oocytes in this study, according to a previous report in reference Citation37. Unfortunately, as the level of active Cdk1 in GV oocytes is too low (data not shown), we cannot test its change in Chk1-depleted or -overexpressed oocytes.
Chk1 was reported to be essential for SAC function and recruitment of BubR1 to the kinetochores.Citation25,Citation38 Different from these reports, we did not observe any defects of SAC function; BubR1 localized correctly to the kinetochores in Chk1-depleted oocytes. One reason for the difference may be the different efficiency of Chk1 depletion.Citation25 We did not detect a specific localization of Chk1 to the kinetochores, which may be another explanation for the different result. A recent study showed that metaphase alignment is not a requirement for anaphase onset,Citation39 and we propose these subtle differences in SAC regulation between meiosis in oocytes and mitosis in somatic cells may also contribute to the different results in our study on mouse oocytes. Chk1 and Chk2 are structurally different, each having a distinctive regulatory domain, but they share many functional similarities and potential redundancies.Citation40 Binding of Chk2 to centrosomes does not require DNA damage, but varies according to cell cycle progression.Citation41 Recently, Chk2 was shown to be necessary for proper mitosis progression.Citation42 Chk2 may compensate for the depletion of Chk1 in mouse oocytes shown in this study. Additionally, Chk1 flox mice were generated,Citation43 and oocyte specific Chk1 conditional knockout mice may be generated to provide in vivo information about functions of Chk1 in meiosis.
Nevertheless, we find that overexpression of Chk1 prevents homologous chromosome segregation and arrests oocytes at MI or earlier stages by activating SAC. These phenotypes resemble that of SAC proteins (such as Bub3, BubR1) or the SAC regulator Spc25 when overexpressed in mouse oocytes,Citation16,Citation17,Citation44 suggesting a possible role for Chk1 in SAC regulation. These data imply that the control of Chk1 quantity is crucial for proper cell cycle progression in mouse oocytes.
In conclusion, our results show that Chk1 is essential for prophase I arrest, and overexpression of Chk1 activates SAC in meiotic mouse oocytes.
Materials and Methods
Oocyte collection and culture.
ICR mice care and handling were performed in accordance with policies proclaimed by the Ethics Committee of the Institute of Zoology, Chinese Academy of Sciences. Oocytes were collected, washed thoroughly and cultured in M2 medium covered with liquid paraffin oil at 37°C in an atmosphere of 5% CO2. Where needed, milrinone (2.5 M) was added to the medium to arrest oocytes at the GV stage.Citation45 The oocytes were collected at different times for immunostaining or western blot.
Chk1 plasmid construction and RNA synthesis.
Total RNA was purified from 200 GV stage oocytes using RNeasy Micro purification kit (Qiagen), and the first strand cDNA was produced with cDNA synthesis kit (Takara). The following two nested primers were used to clone the full-length of Chk1 cDNA by PCR. F1: TTG TCG CTG TGC TTG GAG, R1: CTG GCT GAA AGA AGT CAA GTG G, F2: GTT GGC CGG CCG ATG GCA GTG CCT TTT GTG, R2: GTT GGC GCG CCT CAT GTA ACA GGA AAC CAA ACC. Chk1 cDNA was cloned into pCS2+-myc6 vector. Then the pCS2+-myc6-Chk1 plasmid or empty pCS2+ plasmid was linearized by SalI and SP6 mMESSAGE mMACHINE® kit (Qiagen) and used to prepare capped RNA (myc6-Chk1 mRNA or myc mRNA). Synthesized capped RNA was purified by RNeasy Micro purification kit (Qiagen).
Immunofluorescent microscopy.
For staining Chk1 or BubR1, oocytes were fixed in 4% paraformaldehyde in PBS (pH 7.4) for 30 min and permeabilized in 0.5% Triton-X-100 at room temperature for 20 min. Then oocytes were blocked in 1% BSA-supplemented PBS for 1 h and incubated with rabbit anti-Chk1 (Bioworld Technology, 1:50), sheep polyclonal anti-BubR1 (Abcam; 1:25) antibodies at 4°C overnight. After washing three times in PBS containing 1% Tween 20 and 0.01% Triton-X 100, oocytes were incubated with an appropriate secondary antibody for 1 h at room temperature. The secondary antibodies used in the test were FITC-anti-rabbit IgG (Zhong Shan Jin Qiao; 1:100) and FITC-anti-sheep IgG (Jackson ImmunoResearch; 1:100). After washing three times, the oocytes were stained with PI (10 g/ml) for 10 min. Lastly, oocytes were mounted on glass slides and viewed under a confocal laser scanning microscope (Zeiss LSM 510).
Western blot.
Oocytes (200) were collected in SDS sample buffer and heated for 5 min at 100°C. The proteins were separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were then blocked in 5% skimmed milk-supplemented TBST for 1 h and incubated with mouse monoclonal anti-Chk1 (Santa Cruz; 1:300), rabbit polyclonal anti-Cdc25A (Abcam; 1:500), mouse monoclonal anti-Cdh1 (Abcam; 1:300), mouse monoclonal anti-Cyclin B1 (Abcam; 1:500) or mouse monoclonal anti-β-actin (Zhong Shan Jin Qiao; 1:1,000) antibodies at 4°C overnight. After washing three times in TBST, the membranes were incubated with appropriate IRDye 680RD or IRDye 800CW conjugated secondary antibodies (LI-COR Biotechnology) at room temperature for 1 h. Following three more washes in TBST, the membranes were examined by Odyssey infrared imaging system (LI-COR Biotechnology).
Microinjection of mRNA and siRNAs.
Microinjection was performed using a Nikon Diaphot ECLIPSE TE 300 (Nikon UK Ltd.) inverted microscope equipped with Narishige MM0202N hydraulic three-dimensional micromanipulators (Narishige Inc.) and completed within 30 min. For overexpression of Chk1, myc6-Chk1 mRNA (2.5 mg/ml) was injected into the cytoplasm of oocytes. Oocytes were maintained in M2 medium containing 2.5 μM milrinone for 2 h before transfer into milrinone-free M2 to allow expression of myc6-Chk1. Myc6 mRNA or H2O (virtually no significant difference between the two) Citation14 was injected as control. The following Chk1 siRNA (GenePharma; 50 M) was injected into the cytoplasm of oocytes to deplete Chk1: 5'-CAA CUU GCU GUG AAU AGA AUtt‑3'.Citation46 The sequence of scrambled siRNA (GenePharma, China; 50 M) was: 5'-UUC UCC GAA CGU GUC ACG Utt-3'. After injection, oocytes were arrested at the GV stage in M2 medium containing 2.5 M milrinone for 24 h to prevent resumption of meiosis. Then oocytes were washed and cultured in milrinone-free M2 medium. Each experiment consisted of three separate and replicate groups, and approximately 100 oocytes were injected in each group. The ratio of GVBD or PB1 extrusion was determined using an inverted optical microscope.
Chromosome spread.
Oocytes were kept in 1% sodium citrate for 20 min at room temperature and fixed with fresh methanol: glacial acetic acid (3:1) on glass slides. PI (10 g/ml) was used to stain chromosomes. The slides were analyzed under a confocal laser scanning microscope (Zeiss LSM 510).
Data analysis.
For each treatment, at least three replicates were performed. Statistical analyses were conducted by analysis of variance. Differences between treated groups were analyzed by ANOVA using SPSS software (SPSS Inc.) followed by Student-Newman-Keuls test. Data are expressed as mean ± SEM, and p < 0.05 is considered significant.
| Abbreviations: | ||
| Chk1 | = | checkpoint kinase 1 |
| GV | = | germinal vesicle |
| GVBD | = | germinal vesicle breakdown |
| Pro-MI | = | first pro-metaphase |
| MI | = | first metaphase |
| MII | = | second metaphase |
| PB1 | = | first polar body |
| SAC | = | spindle assembly checkpoint |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Shi-Wen Li and Hua Qin for their technical assistance. This work was supported by Major Basic Research Program (2012CB944404, 2011CB944501) and National Natural Science Foundation of China (30930065) to Q.Y.S.
Note
Supplemental materials can be found at: www.landesbioscience.com/journals/cc/article/20279
References
- Homer H, Gui L, Carroll J. A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science 2009; 326:991 - 4; http://dx.doi.org/10.1126/science.1175326; PMID: 19965510
- Jones KT. Anaphase-promoting complex control in female mouse meiosis. Results Probl Cell Differ 2011; 53:343 - 63; http://dx.doi.org/10.1007/978-3-642-19065-0_15; PMID: 21630152
- Solc P, Schultz RM, Motlik J. Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol Hum Reprod 2010; 16:654 - 64; http://dx.doi.org/10.1093/molehr/gaq034; PMID: 20453035
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 2006; 7:644 - 56; http://dx.doi.org/10.1038/nrm1988; PMID: 16896351
- Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci 2005; 30:630 - 41; http://dx.doi.org/10.1016/j.tibs.2005.09.005; PMID: 16236519
- Solc P, Saskova A, Baran V, Kubelka M, Schultz RM, Motlik J. CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev Biol 2008; 317:260 - 9; http://dx.doi.org/10.1016/j.ydbio.2008.02.028; PMID: 18367163
- Tripathi A, Kumar KV, Chaube SK. Meiotic cell cycle arrest in mammalian oocytes. J Cell Physiol 2010; 223:592 - 600; PMID: 20232297
- Sun QY, Miao YL, Schatten H. Towards a new understanding on the regulation of mammalian oocyte meiosis resumption. Cell Cycle 2009; 8:2741 - 7; http://dx.doi.org/10.4161/cc.8.17.9471; PMID: 19717979
- Dai Y, Grant S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin Cancer Res 2010; 16:376 - 83; http://dx.doi.org/10.1158/1078-0432.CCR-09-1029; PMID: 20068082
- Carrassa L, Damia G. Unleashing Chk1 in cancer therapy. Cell Cycle 2011; 10:2121 - 8; http://dx.doi.org/10.4161/cc.10.13.16398; PMID: 21610326
- Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 2004; 73:39 - 85; http://dx.doi.org/10.1146/annurev.biochem.73.011303.073723; PMID: 15189136
- Takai H, Tominaga K, Motoyama N, Minamishima YA, Nagahama H, Tsukiyama T, et al. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(-/-) mice. Genes Dev 2000; 14:1439 - 47; PMID: 10859163
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 2007; 8:379 - 93; http://dx.doi.org/10.1038/nrm2163; PMID: 17426725
- Wassmann K, Niault T, Maro B. Metaphase I arrest upon activation of the Mad2-dependent spindle checkpoint in mouse oocytes. Curr Biol 2003; 13:1596 - 608; http://dx.doi.org/10.1016/j.cub.2003.08.052; PMID: 13678590
- Homer HA, McDougall A, Levasseur M, Yallop K, Murdoch AP, Herbert M. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev 2005; 19:202 - 7; http://dx.doi.org/10.1101/gad.328105; PMID: 15655110
- Li M, Li S, Yuan J, Wang ZB, Sun SC, Schatten H, et al. Bub3 is a spindle assembly checkpoint protein regulating chromosome segregation during mouse oocyte meiosis. PLoS One 2009; 4:e7701; http://dx.doi.org/10.1371/journal.pone.0007701; PMID: 19888327
- Wei L, Liang XW, Zhang QH, Li M, Yuan J, Li S, et al. BubR1 is a spindle assembly checkpoint protein regulating meiotic cell cycle progression of mouse oocyte. Cell Cycle 2010; 9:1112 - 21; http://dx.doi.org/10.4161/cc.9.6.10957; PMID: 20237433
- Hached K, Xie SZ, Buffin E, Cladière D, Rachez C, Sacras M, et al. Mps1 at kinetochores is essential for female mouse meiosis I. Development 2011; 138:2261 - 71; http://dx.doi.org/10.1242/dev.061317; PMID: 21558374
- Zhang D, Li M, Ma W, Hou Y, Li YH, Li SW, et al. Localization of mitotic arrest deficient 1 (MAD1) in mouse oocytes during the first meiosis and its functions as a spindle checkpoint protein. Biol Reprod 2005; 72:58 - 68; http://dx.doi.org/10.1095/biolreprod.104.032987; PMID: 15342357
- Yin S, Wang Q, Liu JH, Ai JS, Liang CG, Hou Y, et al. Bub1 prevents chromosome misalignment and precocious anaphase during mouse oocyte meiosis. Cell Cycle 2006; 5:2130 - 7; http://dx.doi.org/10.4161/cc.5.18.3170; PMID: 16969117
- McGuinness BE, Anger M, Kouznetsova A, Gil-Bernabé AM, Helmhart W, Kudo NR, et al. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr Biol 2009; 19:369 - 80; http://dx.doi.org/10.1016/j.cub.2009.01.064; PMID: 19249208
- Leland S, Nagarajan P, Polyzos A, Thomas S, Samaan G, Donnell R, et al. Heterozygosity for a Bub1 mutation causes female-specific germ cell aneuploidy in mice. Proc Natl Acad Sci U S A 2009; 106:12776 - 81; http://dx.doi.org/10.1073/pnas.0903075106; PMID: 19617567
- Sun SC, Kim NH. Spindle assembly checkpoint and its regulators in meiosis. Hum Reprod Update 2012; 18:60 - 72; http://dx.doi.org/10.1093/humupd/dmr044; PMID: 22086113
- Tang J, Erikson RL, Liu X. Checkpoint kinase 1 (Chk1) is required for mitotic progression through negative regulation of polo-like kinase 1 (Plk1). Proc Natl Acad Sci U S A 2006; 103:11964 - 9; http://dx.doi.org/10.1073/pnas.0604987103; PMID: 16873548
- Zachos G, Black EJ, Walker M, Scott MT, Vagnarelli P, Earnshaw WC, et al. Chk1 is required for spindle checkpoint function. Dev Cell 2007; 12:247 - 60; http://dx.doi.org/10.1016/j.devcel.2007.01.003; PMID: 17276342
- Peddibhotla S, Lam MH, Gonzalez-Rimbau M, Rosen JM. The DNA-damage effector checkpoint kinase 1 is essential for chromosome segregation and cytokinesis. Proc Natl Acad Sci U S A 2009; 106:5159 - 64; http://dx.doi.org/10.1073/pnas.0806671106; PMID: 19289837
- Nakajo N, Oe T, Uto K, Sagata N. Involvement of Chk1 kinase in prophase I arrest of Xenopus oocytes. Dev Biol 1999; 207:432 - 44; http://dx.doi.org/10.1006/dbio.1998.9178; PMID: 10068474
- Miles DC, van den Bergen JA, Sinclair AH, Western PS. Regulation of the female mouse germ cell cycle during entry into meiosis. Cell Cycle 2010; 9:408 - 18; http://dx.doi.org/10.4161/cc.9.2.10691; PMID: 20023406
- Duncan FE, Chiang T, Schultz RM, Lampson MA. Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol Reprod 2009; 81:768 - 76; http://dx.doi.org/10.1095/biolreprod.109.077909; PMID: 19553597
- Luo Y, Rockow-Magnone SK, Kroeger PE, Frost L, Chen Z, Han EK, et al. Blocking Chk1 expression induces apoptosis and abrogates the G2 checkpoint mechanism. Neoplasia 2001; 3:411 - 9; http://dx.doi.org/10.1038/sj.neo.7900175; PMID: 11687952
- Krämer A, Mailand N, Lukas C, Syljuåsen RG, Wilkinson CJ, Nigg EA, et al. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat Cell Biol 2004; 6:884 - 91; http://dx.doi.org/10.1038/ncb1165; PMID: 15311285
- Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell 2005; 7:193 - 204; http://dx.doi.org/10.1016/j.ccr.2005.01.009; PMID: 15710331
- Tibelius A, Marhold J, Zentgraf H, Heilig CE, Neitzel H, Ducommun B, et al. Microcephalin and pericentrin regulate mitotic entry via centrosome-associated Chk1. J Cell Biol 2009; 185:1149 - 57; http://dx.doi.org/10.1083/jcb.200810159; PMID: 19546241
- Merry C, Fu K, Wang J, Yeh IJ, Zhang Y. Targeting the checkpoint kinase Chk1 in cancer therapy. Cell Cycle 2010; 9:279 - 83; http://dx.doi.org/10.4161/cc.9.2.10445; PMID: 20023404
- Xiao Z, Chen Z, Gunasekera AH, Sowin TJ, Rosenberg SH, Fesik S, et al. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J Biol Chem 2003; 278:21767 - 73; http://dx.doi.org/10.1074/jbc.M300229200; PMID: 12676925
- Bartek J, Lukas J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol 2001; 13:738 - 47; http://dx.doi.org/10.1016/S0955-0674(00)00280-5; PMID: 11698191
- Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 2008; 134:256 - 67; http://dx.doi.org/10.1016/j.cell.2008.05.043; PMID: 18662541
- Carrassa L, Sanchez Y, Erba E, Damia G. U2OS cells lacking Chk1 undergo aberrant mitosis and fail to activate the spindle checkpoint. J Cell Mol Med 2009; 13:8A 1565 - 76; PMID: 19778378
- Nagaoka SI, Hodges CA, Albertini DF, Hunt PA. Oocyte-specific differences in cell-cycle control create an innate susceptibility to meiotic errors. Curr Biol 2011; 21:651 - 7; http://dx.doi.org/10.1016/j.cub.2011.03.003; PMID: 21497085
- Ng CP, Lee HC, Ho CW, Arooz T, Siu WY, Lau A, et al. Differential mode of regulation of the checkpoint kinases CHK1 and CHK2 by their regulatory domains. J Biol Chem 2004; 279:8808 - 19; http://dx.doi.org/10.1074/jbc.M312215200; PMID: 14681223
- Golan A, Pick E, Tsvetkov L, Nadler Y, Kluger H, Stern DF. Centrosomal Chk2 in DNA damage responses and cell cycle progression. Cell Cycle 2010; 9:2647 - 56; http://dx.doi.org/10.4161/cc.9.13.12121; PMID: 20581449
- Stolz A, Ertych N, Kienitz A, Vogel C, Schneider V, Fritz B, et al. The CHK2-BRCA1 tumour suppressor pathway ensures chromosomal stability in human somatic cells. Nat Cell Biol 2010; 12:492 - 9; http://dx.doi.org/10.1038/ncb2051; PMID: 20364141
- Lam MH, Liu Q, Elledge SJ, Rosen JM. Chk1 is haploinsufficient for multiple functions critical to tumor suppression. Cancer Cell 2004; 6:45 - 59; http://dx.doi.org/10.1016/j.ccr.2004.06.015; PMID: 15261141
- Sun SC, Lee SE, Xu YN, Kim NH. Perturbation of Spc25 expression affects meiotic spindle organization, chromosome alignment and spindle assembly checkpoint in mouse oocytes. Cell Cycle 2010; 9:4552 - 9; http://dx.doi.org/10.4161/cc.9.22.13815; PMID: 21084868
- Tsafriri A, Chun SY, Zhang R, Hsueh AJ, Conti M. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol 1996; 178:393 - 402; http://dx.doi.org/10.1006/dbio.1996.0226; PMID: 8812137
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005; 436:1186 - 90; http://dx.doi.org/10.1038/nature03884; PMID: 15995699