Abstract
Why doesn’t the F19A mutant of p53 bind to MDM2? Binding thermodynamics have suggested that the loss of packing interactions upon mutating Phe into Ala sidechain results in destabilizing the binding free energy between p53 and MDM2. Does this mutation also modulate the initial recognition between p53 and MDM2? We look at atomistic computer simulations of the process of the initial encounter between wild type p53 peptide and its F19A mutant with the N-terminal domain of MDM2. These simulations show that binding is characterized by a complex multistep process. It starts with the capture of F19 of wild type p53 by certain residues in the MDM2 binding pocket. This initial step anchors the peptide onto the surface of MDM2, and with the consequent reduction in the search space of the peptide, the peptide docks into the partially occluded surface of MDM2. This is similar to a crack forming in an otherwise occluded hydrophobic cavity in MDM2, and the peptide, docked through F19, modulates the propagation of this crack, which subsequently results in the stepwise docking of the rest of the peptide through insertions of W23 and L26. The lack of the bulky sidechain of F in the F19A mutant results in the absence of the initial “grasp” complex, and hence the mutant peptide diffuses randomly on the surface of MDM2 without binding. This is the first such demonstration of the possibility that a “kinetic” effect may partly underlie the destabilized thermodynamics of binding of F19A and is a feature that appears to be conserved in evolution. The observations by Wallace et al. (Mol Cell 2006; 23:251–63) that despite the inability of F19A to bind at the N-terminal domain of MDM2, it gets ubiquitinated, can now be partly understood based on a mechanism whereby the occupation of the binding pocket by ligands/peptides induces, via crack propagation and the dynamics of gatekeeper Y100, the ubiquitination signal for interactions between the acidic domain of MDM2 and the DNA binding domain of p53.
The ubiquitin ligase MDM2 negatively regulates the tumor suppressor protein p53 in normal cells by binding to it, blocking its function as a transcription factor and targeting it for degradation by promoting its ubiquitin modification.Citation1 In stressed cells, the MDM2-p53 complex formation is inhibited by posttranslational changes such as phosphorylation. This releases p53, which then activates a coordinated response to trigger either cell repair processes or apoptosis.Citation1 The interaction between the transactivation (TA) domain of p53 and the p53 binding domain of MDM2 under chemical equilibrium conditions has been well characterized by experiments and computations;Citation2-Citation4 three amino acid side chains F19, W23 and L26 have been shown to make the most critical contribution to the association. These three residues of p53 lie on the hydrophobic face of an amphipathic helix () and embed into a hydrophobic pocket in MDM2. There is a limited degree of tolerance for substitutions in these three positions, with L26 being the most tolerant.Citation5 It has been shown that the mutation F19A renders the peptide incapable of binding MDM2 and is thus used as a control in experiments;Citation6,Citation7 for example, the F19A mutation makes p53 resistant to MDM2-mediated degradation.Citation8 Indeed, the loss of packing interactions between p53 and MDM2 when the bulky sidechain of F19 is reduced to a smaller sidechain in A19 will result in destabilized interactions in the complex and, hence, reduce their affinity for each other. However, there is a possibility that such mutations may also impact the initial recognition between the two species prior to final complexation; indeed, computational studies have lead to the development of hypotheses such as the identification of a fly-casting mechanism.Citation9-Citation11 There is increasing recognition of the complex choreography that characterizes the interaction between a peptide, particularly the ones that are disordered in solution, and a protein domain. Experimental and computational studies are beginning to reveal that these interactions are multi-step processes, starting from an initial encounter complexCitation12-Citation15 followed by some degree of folding/binding/induced fit.Citation9,Citation10,Citation16-Citation22 In the case of the interaction between p53 and MDM2, the story is complicated by the observation that the p53 binding pocket of MDM2, so “open” in the crystal structure of the complex between p53 and MDM2, is actually occluded in the apo state in solution as revealed by NMR.Citation23 This suggests that the binding pocket is by itself not in a conformation suitable for peptide binding, and that binding must involve a combination of the peptide docking to some partially open pocket in the conformational ensemble of MDM2 molecules followed by “induced opening” of the pocket and subsequent “induced fit” of the peptide into this pocket.Citation12 Unfortunately, this level of atomistic detail along the pathway of binding is not yet amenable to experimental determination. Recent developments in computer hardware and simulations have begun to enable the elucidation of atomistically detailed pathways of the binding of ligands to proteins but require very long simulations that are not easily accessible.Citation24-Citation26
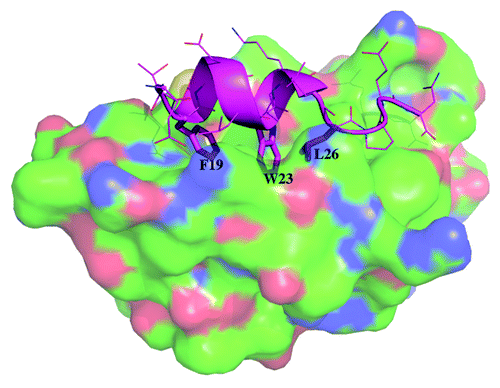
Figure 1. Crystal structure of MDM2 (surface with carbon in green, nitrogen in blue and oxygen in red) and wild type p53 (cartoon in cyan with the sidechains of the 3 critical residues F19, W23 and L26 shown in sticks while the other sidechains are shown in lines) taken from PDB code 1YCR.
To address the mechanism of the lack of binding of the F19A mutant of p53 to MDM2, we first study the thermodynamics of the interaction between wild-type p53 and MDM2 and between the F19A peptide and MDM2; we assume that the description of the interaction of the peptide fragment of wild-type p53 with MDM2 as seen in the crystal structureCitation3 is a good description of their actual interaction. We further assume that if the F19A mutant peptide were to bind MDM2, it would adopt the same overall conformation in its complex as seen for the wild-type peptide. The energetics of such complexation are computed carrying out molecular dynamics (MD) simulations of the two complexes and computing the binding affinities using the MMGBSA protocol in a manner that we have used for analyzing the p53-MDM2 system elsewhere.Citation13,Citation27 These studies have suggested that the loss of the hydrophobic interactions and extensive contacts that are incurred by replacing the large Phe sidechain with the much smaller Ala sidechain leads to a large loss (~14 kcal/mol) of binding energy between the peptide and MDM2 (Citation27 and ) arising, as expected, from the van der Waals component. This reduction in affinity has been reported from several experiments.Citation6,Citation28 This clearly suggests that the lack of binding of F19A to MDM2 may possibly arise from a significantly reduced residence time of the peptide in its bound state.Citation29
Table 1. Computed binding energetics (all values in kcal/mol) of the wild type p53 peptide with MDM2
Table 2. Computed binding energetics (all values in kcal/mol) of the F19A p53 peptide with MDM2
However, the above process describes the state of the peptide after it has formed a complex with MDM2, albeit a rather short-lived one. But complex formation must be preceded by initial recognition events that clearly will play a major role in complexation. In earlier work, where we had explored the docking of the wild-type p53 peptide to MDM2, we had noticed that long-range electrostatics brought the two species together, but at close range van der Waals interactions dominated and drove binding.Citation13 This appeared to manifest in a phase where the peptide and MDM2 engage in exploration of each other’s surfaces, resulting in the formation of transient complexes coupled to stepwise docking. Hence it is not surprising that in the F19A mutant, the loss of the larger sidechain of F19 would manifest in altered modulation of the protein and peptide structures by each other. Such dynamical interactions leading to complex formation have been proposed for a variety of systems.Citation30,Citation31 We now examine the process of binding of the wild-type and F19A mutants to the N-terminal domain of Mdm2 in some detail.
During the initial approach of p53 to MDM2 a large cationic potential on the surface of MDM2 that lies at the N-terminal end of the docked p53 appears to exert a pull on the p53 peptide. During this process of association, it was apparent that the p53 peptide first engaged MDM2 physically by the docking of the F19 sidechain into the binding pocket of MDM2. To pursue these further, simulations were performed to examine the “approach” of p53 and MDM2 to each other for both the wild-type and for the F19A peptide. The calculations were performed for both the open state of MDM2 as extracted from the crystal structure of the complex between MDM2 and p53 (PDB code 1YCR) and for a closed state of MDM2 that was extracted from the NMR structure of apo MDM2 (PDB code 1Z1M);Citation23 this was done because the hydrophobic binding site of p53 in its apo state is not as accessible as it is in the crystal structure of its complex with p53. Although the sampling covers only a small subspace of the large set of encounter complexes, the studies, nevertheless, yield clues regarding the intricate manner in which the two surfaces modulate each other as they “embrace” and how this induces the transition of the binding pocket of MDM2 from a closed to an open state. The docking of the wild-type p53 into MDM2 appears to proceed by the docking of the sidechain of F19 ( and ii) and is further strengthened by the interaction between E17 and the cationic region of MDM2 around K70 (-iii). This appears to firmly anchor the peptide onto the surface, albeit asymmetrically, through its N-terminal end, even as F19 nestles between M62, Y67 and V75 in a hydrophobic clamp constrained by a hydrogen bond between the sidechain of Q72 and the amide of the F19 backbone (-iv). The initial docking and sequestration of F19 into the hydrophobic cluster of M62, Y67, V75 and Q72 reduces the search space for the rest of the peptide. Local rearrangements and partial unfolding or opening of the binding site is accompanied by insertion of W23 (the W23 is initially anchored between the side chains of F55 and Q59 with an Hbond between the carbonyl of the side chain of Q59 and the NH on the side chain of W23) as it is attracted to (or eased into) the hydrophobic pocket and, subsequently, by L26 (–iv), showing the gradual opening of the binding site starting from an occluded state and leading approximately to the conformation seen in the p53-MDM2 crystal structureCitation3 (also see Movie1a-wt and Movie1b-wt). In addition, there are occasional encounters where the F19 and L26 appear to dock initially followed by embedding of W23. Earlier studiesCitation12 had shown that the gatekeeper residue Tyr100 occupies the position in the apo state that would be partly occupied by L26. These two residues compete for this space as p53 “wriggles” into the binding pocket (see Movie M2a and M2c at http://web.bii.a-star.edu.sg/~shubhragd/paper_movies/additional/).Citation12 However with the F19 and W23 anchored into the binding pocket, L26 slowly embeds into the binding pocket and nudges the side chain of Y100 out of the binding pocket. Y100 is stabilized with a network of Hbonds to the C terminus of the p53 peptide in 1YCR (and likely by the carbonyl in the longer p53) and the side chain of Y104. In the docking of the p53 peptide, this movement of Y100 is the last phase of the crack propagating along the binding pocket. Indeed, it is this very residue whose dynamics intimately control the size of the binding pocket.Citation32-Citation34
Figure 2. (a-i)-(a-iv) show representative snapshots from the trajectories of wild type p53 peptide (cyan) approaching MDM2 (green). F19 and W23, the two important side chains of p53 have been shown with partially transparent spheres that clearly shows the initial anchoring of F19 followed by incorporation of W23 leading to complex formation. The animations can also be seen in movies included as Supporting information. (b-i)-(b-iv) shows the representative snapshots for the F19A mutant p53 peptide (cyan) approaching MDM2 (green) and its failure to anchor is evident.
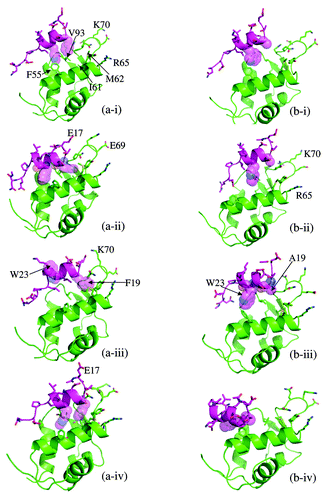
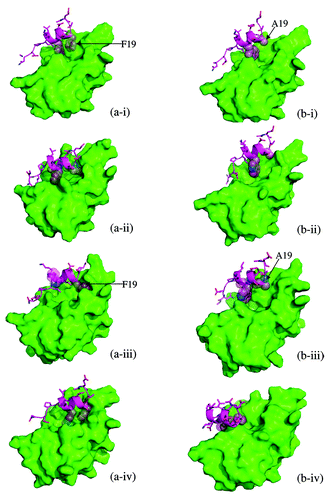
Figure 3. The snapshots shown in are shown with the surface representation of MDM2 to highlight the development of the binding groove and the anchoring.
We next repeated the studies with the F19A mutant of the p53 peptide, starting from the same relative orientations as for the wild-type peptide. We assume that the F19A mutation does not affect the electrostatic complementarity of the two partners,Citation12,Citation35 since this is a hydrophobic to hydrophobic mutation. Simulations reveal that as for the wild-type p53, F19A is initially electrostatically drawn toward MDM2, especially with the attraction between E17 of F19A and the region around K70 of MDM2 (). However, in the absence of the longer hydrophobic sidechain of F19 in F19A, the cluster of side chains M62, Y67, V75 and Q72 are unable to “grasp” the side chain at position 19 (-ii and iii) and “anchor” the peptide, with the result that the peptide appears to slide on the surface of MDM2 in a seemingly random manner (-iv). The corresponding surface figures are shown in –iv and Movie2a-f19a and Movie2b-f19a). The calculations were repeated for the wild-type p53 and the F19A using as target the apo state of MDM2 (taken from the NMR structure), and the pattern we see is the same; note that the apo state of MDM2 as derived from the NMR structure has the p53 binding pocket fairly occluded to begin with. Hence the observation that the wild-type p53 actually anchors to apo MDM2 through F19 followed by the multistep insertion that includes W23 followed by the rest of the peptide lends further support to our hypothesis.
We hypothesize that F19 is not only responsible for thermodynamic stabilization of the peptide, but also critical for initiating docking to MDM2. The current simulations suggest that the nucleation of this complex multistep peptide binding process starts with the anchoring of F19. Experimental reports have shown that complexes involving F19A undergo loss of stability to the extent of ~50%.Citation28 It has also been shown that that the F19A mutant of p53 does not respond to ubiquitination by MDM2Citation36 and appears to need binding at the p53-binding site. Our simulations suggest that either the F19A mutant does not bind to MDM2, or else the residence time is too short for the signal to transfer allosterically to the ubiquitination site. This study paves the way for testing the validity of this hypothesis by generating mutants that can be defective at different stages of this step-wise process and perhaps give higher-resolution insights into signaling mechanisms. For example, the M62A mutant should disable the initial capture event, while the F55A/Q59A mutants (single or double) should arrest the peptide in a state where it is captured only at F19.
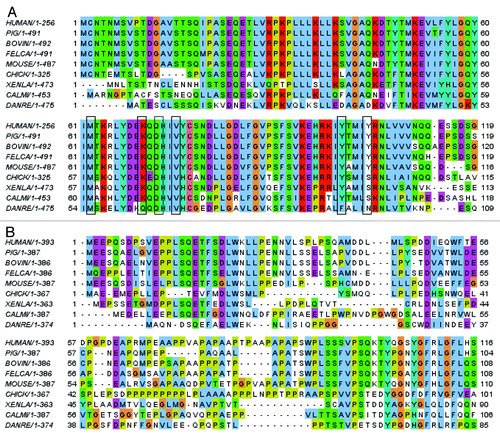
This process of local “unfolding” of the binding pocket that is initiated/activated by the formation of an initial “defect” or “fault” in the binding pocket of MDM2 by insertion of F19 appears similar to the process of crack propagation so familiar in materials research. As the crack opens further upon the initial F19 insertion, the anchored peptide now enables W23/L26 to insert into the propagating crack, thus stabilizing it and catalyzing the widening process. The fact that residues M62, Y67, K70, Q72 and V75 are conserved in MDM2 () and that E17, F19, W23 and L26 are conserved in p53 () further lends support to this hypothesis that, indeed, this mechanism of initial encounter between p53 and MDM2 and subsequent binding may be conserved in evolution; there are differences in some fishes, with an I in place of M62, and in zebrafish, K70 is replaced by Q; however, the interactions of the incoming peptides involve the hydrophobic regions of these side chains, suggesting that the overall patterns of stepwise binding will be conserved, albeit with differences in the kinetics of capture. The importance of these residues was also highlighted in an early computational studyCitation4 of the thermodynamics of p53-MDM2 interactions, where they showed that Ala scan of M62, Y67, Q72 leads to a substantial destabilization of the interactions.
Figure 4. (A) Sequence alignment of the p53 binding domain of MDM2 from different species. (B) Sequence alignment of the N-terminal domain of p53 from different species.
What implications may this have for the general control (transactivation repression/degradation) of p53 by MDM2? A comprehensive study with a very provocatively appealing hypothesis was presented by Kathryn Ball and coworkers.Citation36 They demonstrated unambiguously that the interaction between p53 and MDM2 in the region we have examined modulates ubiquitination. This interaction triggers a signal that modulates the interaction between the acidic domain of MDM2 and a region in the DNA binding domain of p53 and leads to ubiquitination of p53.Citation37 They find that when the peptide PPL SQE TFS DLW KLL P (BOX1 peptide) binds to MDM2, ubiquitination of p53 is stronger than when the peptide SQE TFS DLW KLL P (12-1 peptide) binds to MDM2, and this, in turn, leads to a ubiquitination signal that is stronger than when nutlin binds. From the series of peptides they tested, it is clear that the maximum ubiquitination occurs when the binding cavity is occupied by the peptide SQE TFS DLW KLL PEN and also that ubiquitination only occurs when the peptides extend to the residue P and beyond. The crystal structure 1YCR (with the N-terminal p53 binding domain of MDM2 complexed to a p53 peptide) shows that P27 of the p53 peptide pushes against Y100 (as a result this points away from the p53 binding cavity), and, together, the PEN region of p53 and Y100–Y104 of MDM2 engage E25 (the end of the 1–24 “lid” region of MDM2); further, the C-terminal end of MDM2 lies at the end of the helix that contains Y100 and Y104. Hence it is clear that modulation of this region by any ligand occupying the p53 binding cavity will affect the conformational dynamics of the lid and of the C terminus of the N-terminal domain of MDM2. The latter links to the acidic domain of MDM2, which, in turn, interacts with the DNA binding domain, modulating ubiquitination. Crystal structures of short peptides, including one that terminates with P (RCSB code 2Z5T),Citation38 reveal the Y100 pointing “in” and so does Y104. Examination of the crystal structure 1RV1Citation39 (nutlin bound to the p53-binding N-terminal domain of MDM2) reveals that nutlin has a hydroxyl group that just extends past the site occupied by L26 in p53; Y100 points “in,” although Y104 points away. MD simulations of nutlin complexed to MDM2,Citation35 and the NMR structure of a nutlin-like molecule bound to MDM2 (RCSB code 1TTV)Citation40 shows that Y100 toggles between the “in” and “out” positions. Thus the evidence from the work of Ball and coworkers, when framed against the available dynamic models, suggests that when Y100 (and possibly Y104) are in the “out” position, an allosteric signal is transmitted that leads to some conformational change, and the associated interaction between the acidic domain of MDM2 and the DNA binding domain of p53 led to ubiquitination. When nutlin occupies the binding pocket, Y100 largely points “in” with a small population of the “out” state. This offers a possible mechanism underlying the observation that ubiquitination of p53 by MDM2 bound to nutlin is attenuated compared with when it is bound to “activating” peptides, such as BOX1 or 12-1. Indeed, this may also explain the low ubiquitination when full-length wild-type p53 is used in their experiments. In simulation studies, we find that the C-terminal end of the p53 peptides, when bound to MDM2, is very flexible,Citation27,Citation41 and it is possible that p53 exists in two states when bound to MDM2—a state where its PEN motif is embedded in MDM2 and pushes against Y100–Y104, leading to ubiquitination, and another state where this segment is free, and as a result, no signal is transmitted. If this model is correct, then the P27S mutant peptide of p53Citation5,Citation27 should provide an interesting test system. Earlier workCitation27 suggested that this peptide can adopt two conformations when bound to MDM2, with similar affinities—a helical conformation and a semi-extended conformation. The former is modulated by Y100 toggled “in” and will not trigger the allosteric signal, while the more extended conformation, with Tyr toggled “out” should lead to ubiquituination.
Together, these models suggest that the transmission of the crack will modulate the toggling of Y100 (and Y104), which, in turn, will modulate ubiquitination. This begins to explain their observation that the F19A mutant p53 is ubiquitinated in the presence of these “activating” peptides. The transactivation domain of F19A p53 cannot be “captured” by the N-terminal domain of MDM2; however, its DNA binding domain can interact with the acidic domain of MDM2. Hence, for the allosteric signal to be transmitted and for ubiquitination to take place, the p53-binding site in the N-terminal domain of MDM2 must be occupied by molecules such as nutlin or “activating” peptides as is seen in the work of Ball and colleagues.Citation36 This occupation of the p53 binding cavity will trigger the allosteric signal, via the Y100–Y104 axis, and lead to ubiquitination of F19A. So for any p53-like protein that interacts with MDM2, the occupation of the p53 binding cavity of the N-terminal domain of MDM2, and the interaction with the DNA binding domain appears to be critical.
These observations suggest that there are at least two factors that control ubiquitination and transactivation repression: (1) the occupancy of the p53-binding site of MDM2, particularly the region near Y100, is essential to transmit the allosteric signal; (2) the kinetics of crack propagation will only modulate the rates of transmission of the allosteric signal. Across species, the variations in the amino acids that constitute the binding pocket will determine the rate at which p53 embeds and, hence, the rate at which the allosteric signal will be transmitted, if at all. Short peptides appear to have a higher affinity for MDM2 and will most likely only stabilize p53, while longer peptides and larger molecules will, in addition, lead to its ubiquitination. It is possible that organisms such as viruses exploit this mechanism and degrade p53 very efficiently.Citation42 It will be interesting to see if the replacement of Y104 in MDM2 by R (R103 is the equivalent residue in MDM4) will attenuate the signal transmission.
So how does this translate into other species such as zebrafish. Zebrafish p53 has the sequence FAE LWE KN (human is FSD LWK LL). In structural models that we built (data not shown), the conformation adopted at the C-terminal end of the peptide, near the Y100–Y104 region (in zebrafish it is F and N, respectively) appears to adopt a helical motif that does not “push” at the F region. Indeed, most mammals appear to have Y-Y, but chicken, opossum and frogs have Y-S, while fishes have F-N/M/T. It is not clear how exactly these amino acids will effect the transmission of the signals, but, nevertheless, we can hypothesize that they will not be very effective. This suggests that suppression of transactivation of p53 in fishes and some mammals would be more dominant than ubiquitination. If our model is right, then partly transforming the human to the zebrafish p53 and MDM2 by making the Y100F and Y104N (with H96P) mutations in human MDM2 together with mutation of human p53 to EFA ELW EKN LII Q should shift the balance from ubiquitination to suppression of transactivation.
In summary, these simulations highlight the complexity of the flexible interactions and modulations that characterize the p53-MDM2 axis. They suggest how species variations in amino acid compositions and associated changes in flexibilities, particularly at the part of MDM2 where the C-terminal end of the transactivation domain of p53 binds, control the kinetics and affinity of the interactions between p53 and MDM2. These, in turn, lead to altered conformational dynamics of the Y100–Y104 region (in human MDM2), which will modulate the balance between suppression of p53 transactivation and its degradation through ubiquitination. Indeed, this suggests a provocative, yet plausible idea that these local dynamics may somehow send pulses of allosteric signals that determine the levels of ubiquitination and hence the stability/activity of p53.Citation22 Finally, this also has implications for designing drugs that occupy the p53-binding cavity of MDM2. Lane and colleaguesCitation43 hypothesize that the interaction between the DNA binding domain and MDM2 is abrogated by proteins such as p19ARF, ribosomal proteins, etc. in cells that are oncogenically stressed, and so the inhibition of the p53-MDM2 interaction at the N-terminal domain by molecules such as nutlin will lead to full stabilization of p53. In contrast, for cells where the DNA binding domain interaction is not inhibited, the size of the ligand that will displace the N-terminal domains of p53 and MDM2 will be critical, as too large a ligand may release p53 but also cause its ubiquitination and degradation.
The idea of deformations and nonlinearity and its relationships to functional motions in proteins is oldCitation44-Citation47 but has not been explored much at the level of local details until recently. There now appears to be increasing appreciation of this phenomenon, as exemplified by the recent identification of fault induction from the generation of a local electric field resulting from ionization of a Glutamic acid buried in a hydrophobic pocket in photoactive yellow protein, which appears to trigger a large amplitude protein quake or activated conformational change;Citation48 unfolding of titin under mechanical stress;Citation49 fracture in protein crystals;Citation50 partial unfolding (cracking) of binding motifs modulating the interactions between kinesin and microtubulesCitation51 and contribution of local unfolding or “cracking” in Adenylate kinase to its functional dynamics;Citation52 most recently, the identification that crack propagation in networks can lead to unfolding.Citation53
Indeed, the electrostatic recognition driving the interacting partners toward each other as we see for p53-MDM2 followed by the local conformational rearrangements is thought to drive many processes in biomolecular interactions and regulations. Combining experiments with developments in simulationsCitation24,Citation54 promises much and has already begun to yield some of these exquisite details. These have, for example, highlighted the complex dynamics of the interactions of p21 and of p27 with cyclin-CDK,Citation15,Citation55 the recognition of Retinoblastoma by viral E7 proteins,Citation56 etc. All these processes will likely involve an initial docking of one element that will, in turn, be associated with the reduction of the search space for subsequent recognition events. The initial docking will be followed by the propagation of “cracks,” which are associated with local unfolding/activation, and these must be responsible for lowering the activation barriers for the subsequent multi-step recognition events.Citation57 A deep understanding of these events will be exceptionally helpful for the design of improved protein-protein interaction inhibitors such as Nutlin and Abbott that are now just entering clinical use.Citation8 They are of special value in the design of optimized peptides as represented for example by recent analysis of hydrocarbon bond “stapled” peptides.Citation58,Citation59
Additional material
Download Zip (31.2 KB)Note
Supplemental movies can be found at:
http://web.bii.a-star.edu.sg/bmad/f19a/movie1a-wt
http://web.bii.a-star.edu.sg/bmad/f19a/movie1b-wt
http://web.bii.a-star.edu.sg/bmad/f19a/movie2a-f19a
http://web.bii.a-star.edu.sg/bmad/f19a/movie2b-f19a
http://web.bii.a-star.edu.sg/~shubhragd/paper_movies/additional/ (RefCitation12)
References
- Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer 2009; 9:862 - 73; http://dx.doi.org/10.1038/nrc2763; PMID: 19935675
- Böttger A, Böttger V, Garcia-Echeverria C, Chène P, Hochkeppel HK, Sampson W, et al. Molecular characterization of the hdm2-p53 interaction. J Mol Biol 1997; 269:744 - 56; http://dx.doi.org/10.1006/jmbi.1997.1078; PMID: 9223638
- Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 1996; 274:948 - 53; http://dx.doi.org/10.1126/science.274.5289.948; PMID: 8875929
- Zhong H, Carlson HA. Computational studies and peptidomimetic design for the human p53-MDM2 complex. Proteins 2005; 58:222 - 34; http://dx.doi.org/10.1002/prot.20275; PMID: 15505803
- Zondlo SC, Lee AE, Zondlo NJ. Determinants of specificity of MDM2 for the activation domains of p53 and p65: proline27 disrupts the MDM2-binding motif of p53. Biochemistry 2006; 45:11945 - 57; http://dx.doi.org/10.1021/bi060309g; PMID: 17002294
- Böttger A, Böttger V, Sparks A, Liu WL, Howard SF, Lane DP. Design of a synthetic Mdm2-binding mini protein that activates the p53 response in vivo. Curr Biol 1997; 7:860 - 9; http://dx.doi.org/10.1016/S0960-9822(06)00374-5; PMID: 9382809
- Li C, Pazgier M, Li C, Yuan W, Liu M, Wei G, et al. Systematic mutational analysis of peptide inhibition of the p53-MDM2/MDMX interactions. J Mol Biol 2010; 398:200 - 13; http://dx.doi.org/10.1016/j.jmb.2010.03.005; PMID: 20226197
- Lane DP, Cheok CF, Lain S. p53-based cancer therapy. Cold Spring Harb Perspect Biol 2010; 2:a001222; http://dx.doi.org/10.1101/cshperspect.a001222; PMID: 20463003
- Chen J. Intrinsically disordered p53 extreme C-terminus binds to S100B(betabeta) through “fly-casting”. J Am Chem Soc 2009; 131:2088 - 9; http://dx.doi.org/10.1021/ja809547p; PMID: 19216110
- Gsponer J, Babu MM. The rules of disorder or why disorder rules. Prog Biophys Mol Biol 2009; 99:94 - 103; http://dx.doi.org/10.1016/j.pbiomolbio.2009.03.001; PMID: 19344736
- Huang Y, Liu Z. Kinetic advantage of intrinsically disordered proteins in coupled folding-binding process: a critical assessment of the “fly-casting” mechanism. J Mol Biol 2009; 393:1143 - 59; http://dx.doi.org/10.1016/j.jmb.2009.09.010; PMID: 19747922
- Dastidar SG, Lane DP, Verma CS. Modulation of p53 binding to MDM2: computational studies reveal important roles of Tyr100. BMC Bioinformatics 2009; 10:6; http://dx.doi.org/10.1186/1471-2105-10-S15-S6; PMID: 19126227
- Dastidar SG. others a. Forces mediating protein-protein interactions: a computational study of p53 “approaching” MDM2. Theor Chem Acc 2010; 125:621; http://dx.doi.org/10.1007/s00214-009-0682-1
- Dunker AK, Uversky VN. Signal transduction via unstructured protein conduits. Nat Chem Biol 2008; 4:229 - 30; http://dx.doi.org/10.1038/nchembio0408-229; PMID: 18347590
- Wang Y, Fisher JC, Mathew R, Ou L, Otieno S, Sublet J, et al. Intrinsic disorder mediates the diverse regulatory functions of the Cdk inhibitor p21. Nat Chem Biol 2011; 7:214 - 21; http://dx.doi.org/10.1038/nchembio.536; PMID: 21358637
- Chen HF. Molecular dynamics simulation of phosphorylated KID post-translational modification. PLoS One 2009; 4:e6516; http://dx.doi.org/10.1371/journal.pone.0006516; PMID: 19654879
- Cortese MS, Uversky VN, Dunker AK. Intrinsic disorder in scaffold proteins: getting more from less. Prog Biophys Mol Biol 2008; 98:85 - 106; http://dx.doi.org/10.1016/j.pbiomolbio.2008.05.007; PMID: 18619997
- Lacy ER, Filippov I, Lewis WS, Otieno S, Xiao L, Weiss S, et al. p27 binds cyclin-CDK complexes through a sequential mechanism involving binding-induced protein folding. Nat Struct Mol Biol 2004; 11:358 - 64; http://dx.doi.org/10.1038/nsmb746; PMID: 15024385
- Shoemaker BA, Portman JJ, Wolynes PG. Speeding molecular recognition by using the folding funnel: the fly-casting mechanism. Proc Natl Acad Sci U S A 2000; 97:8868 - 73; http://dx.doi.org/10.1073/pnas.160259697; PMID: 10908673
- Sugase K, Dyson HJ, Wright PE. Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature 2007; 447:1021 - 5; http://dx.doi.org/10.1038/nature05858; PMID: 17522630
- Trizac E, Levy Y, Wolynes PG. Capillarity theory for the fly-casting mechanism. Proc Natl Acad Sci U S A 2010; 107:2746 - 50; http://dx.doi.org/10.1073/pnas.0914727107; PMID: 20133683
- Verkhivker GM, Bouzida D, Gehlhaar DK, Rejto PA, Freer ST, Rose PW. Simulating disorder-order transitions in molecular recognition of unstructured proteins: where folding meets binding. Proc Natl Acad Sci U S A 2003; 100:5148 - 53; http://dx.doi.org/10.1073/pnas.0531373100; PMID: 12697905
- Uhrinova S, Uhrin D, Powers H, Watt K, Zheleva D, Fischer P, et al. Structure of free MDM2 N-terminal domain reveals conformational adjustments that accompany p53-binding. J Mol Biol 2005; 350:587 - 98; http://dx.doi.org/10.1016/j.jmb.2005.05.010; PMID: 15953616
- Buch I, Giorgino T, De Fabritiis G. Complete reconstruction of an enzyme-inhibitor binding process by molecular dynamics simulations. Proc Natl Acad Sci U S A 2011; 108:10184 - 9; http://dx.doi.org/10.1073/pnas.1103547108; PMID: 21646537
- Dror RO, Arlow DH, Borhani DW, Jensen MO, Piana S, Shaw DE. Identification of two distinct inactive conformations of the beta2-adrenergic receptor reconciles structural and biochemical observations. Proc Natl Acad Sci U S A 2009; 106:4689 - 94; http://dx.doi.org/10.1073/pnas.0811065106; PMID: 19258456
- Shaw DE, Maragakis P, Lindorff-Larsen K, Piana S, Dror RO, Eastwood MP, et al. Atomic-level characterization of the structural dynamics of proteins. Science 2010; 330:341 - 6; http://dx.doi.org/10.1126/science.1187409; PMID: 20947758
- Dastidar SG, Lane DP, Verma CS. Multiple peptide conformations give rise to similar binding affinities: molecular simulations of p53-MDM2. J Am Chem Soc 2008; 130:13514 - 5; http://dx.doi.org/10.1021/ja804289g; PMID: 18800837
- Liu Z, Olejniczak ET, Fesik SW. Over-expression of the human MDM2 p53 binding domain by fusion to a p53 transactivation peptide. Protein Expr Purif 2004; 37:493 - 8; http://dx.doi.org/10.1016/j.pep.2004.06.036; PMID: 15358376
- Schon O, Friedler A, Bycroft M, Freund SM, Fersht AR. Molecular mechanism of the interaction between MDM2 and p53. J Mol Biol 2002; 323:491 - 501; http://dx.doi.org/10.1016/S0022-2836(02)00852-5; PMID: 12381304
- Medina M, Abagyan R, Gómez-Moreno C, Fernandez-Recio J. Docking analysis of transient complexes: interaction of ferredoxin-NADP+ reductase with ferredoxin and flavodoxin. Proteins 2008; 72:848 - 62; http://dx.doi.org/10.1002/prot.21979; PMID: 18260112
- Waas WF, Dalby KN. Transient protein-protein interactions and a random-ordered kinetic mechanism for the phosphorylation of a transcription factor by extracellular-regulated protein kinase 2. J Biol Chem 2002; 277:12532 - 40; http://dx.doi.org/10.1074/jbc.M110523200; PMID: 11812784
- Buolamwini JK, Addo J, Kamath S, Patil S, Mason D, Ores M. Small molecule antagonists of the MDM2 oncoprotein as anticancer agents. Curr Cancer Drug Targets 2005; 5:57 - 68; http://dx.doi.org/10.2174/1568009053332672; PMID: 15720190
- Khoury K, Popowicz GM, Holak TA, Dömling A. The p53-MDM2/MDMX axis – A chemotype perspective. Med Chem Commun 2011; 2:246 - 60; http://dx.doi.org/10.1039/c0md00248h
- Nicholson J, Hupp TR. The molecular dynamics of MDM2. Cell Cycle 2010; 9:1878 - 81; http://dx.doi.org/10.4161/cc.9.10.11597; PMID: 20436290
- Joseph TL, Madhumalar A, Brown CJ, Lane DP, Verma CS. Differential binding of p53 and nutlin to MDM2 and MDMX: computational studies. Cell Cycle 2010; 9:1167 - 81; http://dx.doi.org/10.4161/cc.9.6.11067; PMID: 20190571
- Wallace M, Worrall E, Pettersson S, Hupp TR, Ball KL. Dual-site regulation of MDM2 E3-ubiquitin ligase activity. Mol Cell 2006; 23:251 - 63; http://dx.doi.org/10.1016/j.molcel.2006.05.029; PMID: 16857591
- Coutts AS, Adams CJ, La Thangue NB. p53 ubiquitination by Mdm2: a never ending tail?. DNA Repair (Amst) 2009; 8:483 - 90; http://dx.doi.org/10.1016/j.dnarep.2009.01.008; PMID: 19217357
- Popowicz GM, Czarna A, Rothweiler U, Szwagierczak A, Krajewski M, Weber L, et al. Molecular basis for the inhibition of p53 by Mdmx. Cell Cycle 2007; 6:2386 - 92; http://dx.doi.org/10.4161/cc.6.19.4740; PMID: 17938582
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004; 303:844 - 8; http://dx.doi.org/10.1126/science.1092472; PMID: 14704432
- Fry DC, Emerson SD, Palme S, Vu BT, Liu CM, Podlaski F. NMR structure of a complex between MDM2 and a small molecule inhibitor. J Biomol NMR 2004; 30:163 - 73; http://dx.doi.org/10.1023/B:JNMR.0000048856.84603.9b; PMID: 15557803
- Liu Y, Lane DP, Verma CS. Systematic mutational analysis of an ubiquitin ligase (MDM2)-binding peptide: computational studies. Theor Chem Acc 2011; 130:1145 - 54; http://dx.doi.org/10.1007/s00214-011-1049-y
- Kashuba E, Yurchenko M, Yenamandra SP, Snopok B, Szekely L, Bercovich B, et al. Epstein-Barr virus-encoded EBNA-5 forms trimolecular protein complexes with MDM2 and p53 and inhibits the transactivating function of p53. Int J Cancer 2011; 128:817 - 25; http://dx.doi.org/10.1002/ijc.25414; PMID: 20473904
- Lane DP, Brown CJ, Verma C, Cheok CF. New insights into p53 based therapy. Discov Med 2011; 12:107 - 17; PMID: 21878188
- Frauenfelder H, Sligar SG, Wolynes PG. The energy landscapes and motions of proteins. Science 1991; 254:1598 - 603; http://dx.doi.org/10.1126/science.1749933; PMID: 1749933
- García AE. Large-amplitude nonlinear motions in proteins. Phys Rev Lett 1992; 68:2696 - 9; http://dx.doi.org/10.1103/PhysRevLett.68.2696; PMID: 10045464
- Kitao A, Hayward S, Go N. Energy landscape of a native protein: jumping-among-minima model. Proteins 1998; 33:496 - 517; http://dx.doi.org/10.1002/(SICI)1097-0134(19981201)33:4<496::AID-PROT4>3.0.CO;2-1; PMID: 9849935
- Miyashita O, Onuchic JN, Wolynes PG. Nonlinear elasticity, proteinquakes, and the energy landscapes of functional transitions in proteins. Proc Natl Acad Sci U S A 2003; 100:12570 - 5; http://dx.doi.org/10.1073/pnas.2135471100; PMID: 14566052
- Xie A, Kelemen L, Hendriks J, White BJ, Hellingwerf KJ, Hoff WD. Formation of a new buried charge drives a large-amplitude protein quake in photoreceptor activation. Biochemistry 2001; 40:1510 - 7; http://dx.doi.org/10.1021/bi002449a; PMID: 11327809
- Marszalek PE, Lu H, Li H, Carrion-Vazquez M, Oberhauser AF, Schulten K, et al. Mechanical unfolding intermediates in titin modules. Nature 1999; 402:100 - 3; http://dx.doi.org/10.1038/47083; PMID: 10573426
- Buehler MJ. Mechanics of Protein Crystals: Atomistic Modeling of Elasticity and Fracture. J Theor and comput. Nanotechnology 2006; 3:670 - 83
- Hyeon C, Onuchic JN. Mechanical control of the directional stepping dynamics of the kinesin motor. Proc Natl Acad Sci U S A 2007; 104:17382 - 7; http://dx.doi.org/10.1073/pnas.0708828104; PMID: 17959770
- Whitford PC, Onuchic JN, Wolynes PG. Energy landscape along an enzymatic reaction trajectory: hinges or cracks?. HFSP J 2008; 2:61 - 4; http://dx.doi.org/10.2976/1.2894846; PMID: 19404472
- de Graff AM, Shannon G, Farrell DW, Williams PM, Thorpe MF. Protein unfolding under force: crack propagation in a network. Biophys J 2011; 101:736 - 44; http://dx.doi.org/10.1016/j.bpj.2011.05.072; PMID: 21806942
- Shan Y, Kim ET, Eastwood MP, Dror RO, Seeliger MA, Shaw DE. How does a drug molecule find its target binding site?. J Am Chem Soc 2011; 133:9181 - 3; http://dx.doi.org/10.1021/ja202726y; PMID: 21545110
- Galea CA, Nourse A, Wang Y, Sivakolundu SG, Heller WT, Kriwacki RW. Role of intrinsic flexibility in signal transduction mediated by the cell cycle regulator, p27 Kip1. J Mol Biol 2008; 376:827 - 38; http://dx.doi.org/10.1016/j.jmb.2007.12.016; PMID: 18177895
- Kinetic recognition of the retinoblastoma tumor suppressor by a specific protein target. Chemes LBSánchez IEde Prat-Gay GJ. Mol Biol 2011; 412:267 - 84; http://dx.doi.org/10.1016/j.jmb.2011.07.015
- Isin EM, Guengerich FP. Multiple sequential steps involved in the binding of inhibitors to cytochrome P450 3A4. J Biol Chem 2007; 282:6863 - 74; http://dx.doi.org/10.1074/jbc.M610346200; PMID: 17200113
- Joseph TL, Lane D, Verma CS. Stapled peptides in the p53 pathway: computer simulations reveal novel interactions of the staples with the target protein. Cell Cycle 2010; 9:4560 - 8; http://dx.doi.org/10.4161/cc.9.22.13816; PMID: 21088491
- Kim YW, Grossmann TN, Verdine GL. Synthesis of all-hydrocarbon stapled α-helical peptides by ring-closing olefin metathesis. Nat Protoc 2011; 6:761 - 71; http://dx.doi.org/10.1038/nprot.2011.324; PMID: 21637196