Abstract
Telomeres are nucleoprotein structures that protect the ends of eukaryotic chromosomes and play important roles in ensuring the genome’s integrity. Telomere length is maintained by complex mechanisms that ensure length homeostasis. Recent work has linked telomere length maintenance to the Tor protein kinases, which are central regulators of cellular growth. Here we summarize these results, which suggest a link between nutrient availability, telomere length maintenance and chronological lifespan.
The TOR (target of rapamycin) proteins are serine/threonine protein kinases which have drawn much attention during the past decade as central regulators of cellular growth, division and survival and as targets for the drug rapamycin (reviewed in refs. Citation1–Citation3). TOR belongs to the family of phosphatydyl inositol‑3 kinase-related kinases (PI3K-like kinases). Other members of this family, like the ATM, ATR and DNA-PK proteins, are well known for their cellular roles in response to DNA damage. TOR proteins share several domains with other PI3K-like kinases, including the HEAT repeats and the FAT and FATC domains, which are thought to be involved in protein-protein interactions. However, unique to the TOR kinases is the FRB (FKBP12-rapamycin binding) domain. This domain is crucial for the binding of rapamycin. The FRB domain binds rapamycin only when the drug is in complex with FKBP12, a small (12 kDa) protein that is highly conserved in evolution from bacteria to humans. The binding of the rapamycin-FKBP12 complex leads to inhibition of a certain subset of TOR-dependent activities, which can lead to beneficial clinical effects. Rapamycin and its derivatives have recently become the focus of many different clinical studies. While rapamycin was initially used in the clinics as an immunosuppressive drug, it is currently in use for treatment against several cancer types and is considered for the treatment of several metabolic and neurological disorders. Furthermore, rapamycin slows aging in a wide range of organisms, including mice.Citation4,Citation5
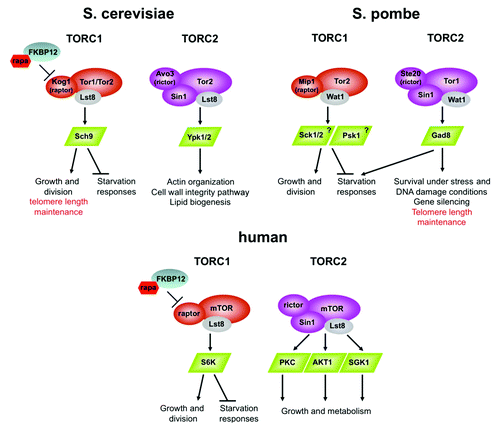
TOR proteins are found in all eukaryotes examined. In the budding yeast Saccharomyces cerevisiae or the fission yeast Schizosaccharomyces pombe, two TOR homologs are present, while a single TOR gene (mTOR) exists in higher eukaryotes. TOR kinases can be found in two structurally and functionally distinct complexes, known as TORC1 and TORC2, which are highly conserved in evolution (). The two complexes share several subunits but differ in critical subunits that determine TORC1- or TORC2-specific activities. In TORC1, TOR is bound to the raptor protein, while in TORC2, TOR is in complex with the rictor and Sin1 proteins. The two TOR complexes exert their effects via phosphorylation and activation of distinct sets of downstream effectors, in particular, via phosphorylation and activation of members of AGC kinases (). Of the two TOR-containing complexes, TORC1 has been more extensively investigated. TORC1 integrates extracellular and intracellular signals that provide information about nutrient availability and energy levels to positively regulate cell mass increase (cellular growth) and cell divisions while inhibiting starvation responses. The growth-related cellular functions of TORC1 are exerted at many different levels but are largely dependent on regulation of protein synthesis and on inhibition of autophagy. The function of TORC1 as a positive growth regulator is highly conserved in evolution. Accordingly, disruption of TORC1 in S. cerevisiae, S. pombe, nematodes, flies or mammalian cells results in cellular phenotypes that highly resemble the phenotype of starved cells (for examples, see refs. Citation6–Citation12). Such a phenotype includes small cells, a marked reduction in protein translation and ribosome biogenesis, transcriptional induction of starvation-specific genes and induction of autophagy. In a wide variety of eukaryotes, most of the cellular functions of TORC1 are inhibited by rapamycin. Accordingly, exposure of S. cerevisiae cells or certain mammalian cell types to the drug results in cessation of growth and the manifestation of a starvation-like phenotype, very similar to disruption of TORC1 activity by gene mutations. Surprisingly, in S. pombe, although TORC1 is essential for growth, rapamycin does not exert a growth-inhibitory effect on wild-type cells.Citation13 Yet, rapamycin inhibits certain TORC1- and TORC2-dependent functions in fission yeast.Citation8,Citation14
Figure 1. Schematic presentation of TORC1 and TORC2 and their downstream AGC-kinase effectors in the budding yeast S. cerevisiae, the fission yeast S. pombe and in humans. Only the critical and highly conserved subunits of TORC1 and TORC2 are presented. The AGC kinases that lie downstream of S. pombe TORC1 are mainly deduced from sequence analysis and their activation by TORC1 awaits further analysis.Citation36
Two recent papers by Ungar et al.Citation15 and Kwan et al. have recently revealed yet another novel and exciting function of TORC1 in budding yeast, which supports its role as a positive regulator of growth and an active inhibitor of starvation responses. Ungar and coworkers demonstrated that a sublethal concentration of rapamycin leads to shortening of telomere length in S. cerevisiae cells. A similar extent of telomere shortening is also observed when cells are grown on low-glucose or low-nitrogen medium, suggesting that starvation signals lead to the short telomeres phenotype. Telomeres are the DNA-protein structures at the end of eukaryotic telomeres. Telomeres are prone to shortening at each replication cycle because of an inherent inability to replicate the ends of linear chromosomes by the replication machinery (the “end replication problem”). Thus, under rapid cell proliferation, a mechanism that adds telomeric DNA repeats is required. The enzyme that carries out the addition of telomeric repeats is telomerase, a ribonucleoprotein enzyme that adds telomeric repeats by reverse transcription activity, using its own RNA subunit as a template. Other mechanisms that affect telomere length also exist. In particular, telomeric repeats can also be preserved, under certain circumstances, by a recombination-dependent mechanism (ALT).Citation17
The length of telomeres is maintained constant by a highly regulated process. In addition to telomerase, other proteins whose effect on telomere length is well-studied are the telomere binding proteins Ku (Yku70/Yku80), the DNA repair complex MRX (Mre11/Rad50/Xrs2), certain DNA damage checkpoint proteins and DNA replication and chromatin-modifying proteins. In addition, genetic screens of the collection of deletion mutant libraries in S. cerevisiae identified about 400 genes that, when mutated, can affect the length of telomeres, either leading to telomere shortening or to increase in telomere length. These are collectively known as TLM (telomere length maintenance) genes.Citation18-Citation21
Having identified shortening of telomeres in response to starvation or rapamycin treatment, Ungar et al. asked what is the molecular mechanism that underlies telomere shortening. Since rapamycin is a potent inhibitor of TORC1 in S. cerevisiae, they examined telomere length in yeast strains mutated for TORC1 components or downstream effectors. Such an approach demonstrated that loss-of-function mutations in Kog1 (raptor homolog), TCO89 (a TORC1 subunit) or the downstream effectors of TORC1, such as the phosphatase Tap42, which acts as a negative regulator or the transcription factors Gln3, all lead to telomere shortening. Importantly, disruption of the S. cerevisiae FKBP12 homolog resulted in resistance to the effect of shortening of telomeres in the presence of rapamycin, further supporting the suggestion that rapamycin affects telomeres by inhibiting TORC1 through the FKBP12-rapamycin toxic complex.Citation15
One of the best-characterized effects of TORC1 on starvation responses is the regulation of nitrogen catabolite repression (NCR). Growth of yeast cells in nitrogen-depleted medium elicits a highly regulated response, in which a large number of genes encoding the transporters and enzymes needed to scavenge poor nitrogen sources are induced. The details of this process have been investigated in several laboratories (reviewed in refs. Citation22–Citation25). Upon nitrogen starvation, TORC1 is inactivated, leading to activation of the phosphatase Sit4, which subsequently causes de-phosphorylation of the transcription factors Gln3 and Gat1. This allows their dissociation from the cytoplasmic protein Ure2, resulting in their entrance into the nucleus. In the nucleus, Gat1 and Gln3 bind the promoters of NCR genes, inducing their transcription and eliciting the nitrogen starvation response. Ungar et al. found discrepancies between telomere length maintenance in gln3- and gat1-null mutant cells, as deletion of GLN3 exhibited normal telomere length, while cells lacking GAT1 have elongated telomeres. Similar inconsistencies between the phenotypes of gln3 and gat1 mutant cells have already been described in references Citation26 and Citation27 and likely reflect elaborate modes of regulation of these two transcription factors.
But how does the NCR response affect telomere length? Ungar and coworkers performed a screen for telomere length maintenance (tlm) mutant strains that are resistant to the telomeric effect of rapamycin. Such mutants may suggest a critical gene required for telomere shortening in the presence of rapamycin. The screen identified yku70 and yku80 as mutant cells that exhibit a short telomere phenotype that is not further affected by rapamycin. This is not due to the short telomere phenotype seen in the absence of rapamycin, as other mutants, such as those defective for the MRX complex or for TEL1 (yeast ATM ortholog), have telomeres of similar size to those of yku70/80 mutants but are further shortened by rapamycin. The suggestion that rapamycin leads to short telomeres by affecting Yku70/80-dependent activity is further supported by the finding that the level of the Yku70 protein is reduced in response to rapamycin. Importantly, reduction of Yku70 is dependent on the integrity of the NCR response. Thus, for example, in the absence of both transcription factors, Gln3 and Gat1, when the NCR response is abolished, cells are resistant to telomere shortening by rapamycin, and the protein levels of Yku70 remain high. Additionally, in the absence of Ure2, when the Gln1 and Gat1 remain constitutively in the nucleus, Yku70 protein levels are constitutively low. Thus, activation of NCR is correlated with low levels of Yku70. What the molecular mechanism is that links the NCR response and Yku70/80 levels is yet to be explored. Since NCR response does not affect the level of transcription of the Yku70/80 genes, the mechanism that regulates Yku70/80 level may act at the level of protein synthesis or protein degradation.
The findings by Kwan et al. also demonstrate that telomere length is regulated by the TORC1-Gln3 pathway. They found that a naturally occurring polymorphism in Bul2, a component of an ubiquitin ligase complex that regulates sorting and expression of amino acid permeases, affects telomere length and chronological life span (CLS). Accordingly, reduced nitrogen availability (amino acid uptake) occurring in cells with functional Bul2 found in a S. cerevisiae vineyard strain led to increase in Gln3 transcriptional activity, which resulted in shorter telomeres and increase in CLS. The data of Kwan and coworkers suggest that Gln3 modulates telomere length maintenance by regulating the expression of Wtm1, an inhibitor of ribonucleotide reductase (RNR). Hence, the decrease in telomere length in ure2 loss-of function mutants reflects limitation of RNR activity and impaired DNA replication.Citation16 Unlike Ungar et al., Kwan and coworkers did not find a role for the Yku70/80 complex in telomere shortening.Citation16 This discrepancy may reflect the use of different yeast strains by the two laboratories. Also, it is possible that different mechanisms work together to link nutrient sensing by TORC1 and telomere length maintenance.
Is regulation of telomere length by TOR conserved in evolution? In an independent study, an increase in telomere length was observed in S. pombe mutant strains that carry loss-of-function mutations in TORC2 or its downstream effector, the AGC kinase Gad8.Citation28 In fission yeast, TORC2 is not required for growth under normal growth conditions, yet its disruption results in pleiotropic defects, in particular, inability to respond to nutrient depletion or to a variety of other stresses, including DNA damage and DNA replication stresses.Citation29,Citation30 Interestingly, in fission yeast, TORC1 and TORC2 oppositely regulate several starvation responses, for example, sexual development and transcriptional activation of several nitrogen starvation-responsive genes.Citation8 Whether S. pombe TORC1 also participates in telomere length maintenance is yet to be determined. Moreover, whether telomere length regulation in higher eukaryotes is subjected to regulation by the TOR signaling and/or by nutritional signals is yet an open question. If this regulation is conserved, the results obtained in yeast may have important consequences for cancer and aging. Due to its central role in cell growth and metabolism, inappropriate upregulation of the TOR pathway has been implicated in various malignancies, including cancers of the colon, breast, liver, brain, stomach, lung and ovary.Citation1-Citation3,Citation31 Several rapamycin derivatives are currently undergoing clinical trials for the treatment of carcinomas, lymphomas and other types of cancers. Since telomere maintenance is essential for cancer cells, telomere shortening by rapamycin may have additional beneficial effects for cancer treatment. In addition, Ungar et al. showed that starvation and rapamycin treatment lead to reduced levels of Ku heterodimer, resulting in nonhomologous end joining (NHEJ) deficiency.Citation15 NHEJ is the main repair mechanism for double-strand breaks in mammals; rapamycin may thus render the cells hypersensitive to DNA damaging agents. In addition, calorie restriction, which has been shown to extend lifespan by inhibiting TOR signaling in several organisms,Citation32-Citation34 may, at the same time, lead to shorter telomeres. As demonstrated by Kwan et al., decrease in amino acid uptake led to an increase in chronological lifespan and a short telomere phenotype. This surprising correlation between short telomeres and CLS does not apply to other short telomere mutants.Citation16 Rather, it appears that the TORC1-Gln3 pathway regulates both telomeres and CLS in response to nutritional signals. Further studies are required to understand the interplay between calorie restriction and telomere length maintenance in determining cellular lifespan.Citation35 Despite the fact that a negative correlation has been found between telomere length and age in humans, it is possible that the TOR kinases may control life span through separate mechanisms.
Acknowledgments
This work was supported by a grant from the Association for International Cancer Research (AICR) to R.W. and grants from the Israel Cancer Research fund and the Israel Cancer Research Foundation to M.K.
References
- Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle 2011; 10:2305 - 16; http://dx.doi.org/10.4161/cc.10.14.16586; PMID: 21670596
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011; 12:21 - 35; http://dx.doi.org/10.1038/nrm3025; PMID: 21157483
- Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol 2011; 23:744 - 55; http://dx.doi.org/10.1016/j.ceb.2011.09.003; PMID: 21963299
- Conn CS, Qian SB. mTOR signaling in protein homeostasis: less is more?. Cell Cycle 2011; 10:1940 - 7; http://dx.doi.org/10.4161/cc.10.12.15858; PMID: 21555915
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009; 460:392 - 5; PMID: 19587680
- Barbet NC, Schneider U, Helliwell SB, Stansfield I, Tuite MF, Hall MN. TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell 1996; 7:25 - 42; PMID: 8741837
- Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem 1998; 273:3963 - 6; http://dx.doi.org/10.1074/jbc.273.7.3963; PMID: 9461583
- Weisman R, Roitburg I, Schonbrun M, Harari R, Kupiec M. Opposite effects of tor1 and tor2 on nitrogen starvation responses in fission yeast. Genetics 2007; 175:1153 - 62; http://dx.doi.org/10.1534/genetics.106.064170; PMID: 17179073
- Matsuo T, Otsubo Y, Urano J, Tamanoi F, Yamamoto M. Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol Cell Biol 2007; 27:3154 - 64; http://dx.doi.org/10.1128/MCB.01039-06; PMID: 17261596
- Long X, Müller F, Avruch J. TOR action in mammalian cells and in Caenorhabditis elegans. Curr Top Microbiol Immunol 2004; 279:115 - 38; http://dx.doi.org/10.1007/978-3-642-18930-2_8; PMID: 14560955
- Meissner B, Boll M, Daniel H, Baumeister R. Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. J Biol Chem 2004; 279:36739 - 45; http://dx.doi.org/10.1074/jbc.M403415200; PMID: 15155758
- Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev 2000; 14:2712 - 24; http://dx.doi.org/10.1101/gad.835000; PMID: 11069888
- Weisman R, Roitburg I, Nahari T, Kupiec M. Regulation of leucine uptake by tor1+ in Schizosaccharomyces pombe is sensitive to rapamycin. Genetics 2005; 169:539 - 50; http://dx.doi.org/10.1534/genetics.104.034983; PMID: 15466417
- Petersen J, Nurse P. TOR signalling regulates mitotic commitment through the stress MAP kinase pathway and the Polo and Cdc2 kinases. Nat Cell Biol 2007; 9:1263 - 72; http://dx.doi.org/10.1038/ncb1646; PMID: 17952063
- Ungar L, Harari Y, Toren A, Kupiec M. Tor complex 1 controls telomere length by affecting the level of Ku. Curr Biol 2011; 21:2115 - 20; http://dx.doi.org/10.1016/j.cub.2011.11.024; PMID: 22169538
- Kwan EX, Foss E, Kruglyak L, Bedalov A. Natural polymorphism in BUL2 links cellular amino acid availability with chronological aging and telomere maintenance in yeast. PLoS Genet 2011; 7:e1002250; http://dx.doi.org/10.1371/journal.pgen.1002250; PMID: 21901113
- Bianchi A, Shore D. How telomerase reaches its end: mechanism of telomerase regulation by the telomeric complex. Mol Cell 2008; 31:153 - 65; http://dx.doi.org/10.1016/j.molcel.2008.06.013; PMID: 18657499
- Askree SH, Yehuda T, Smolikov S, Gurevich R, Hawk J, Coker C, et al. A genome-wide screen for Saccharomyces cerevisiae deletion mutants that affect telomere length. Proc Natl Acad Sci U S A 2004; 101:8658 - 63; http://dx.doi.org/10.1073/pnas.0401263101; PMID: 15161972
- Gatbonton T, Imbesi M, Nelson M, Akey JM, Ruderfer DM, Kruglyak L, et al. Telomere length as a quantitative trait: genome-wide survey and genetic mapping of telomere length-control genes in yeast. PLoS Genet 2006; 2:e35; http://dx.doi.org/10.1371/journal.pgen.0020035; PMID: 16552446
- Shachar R, Ungar L, Kupiec M, Ruppin E, Sharan R. A systems-level approach to mapping the telomere length maintenance gene circuitry. Mol Syst Biol 2008; 4:172; http://dx.doi.org/10.1038/msb.2008.13; PMID: 18319724
- Yosef N, Ungar L, Zalckvar E, Kimchi A, Kupiec M, Ruppin E, et al. Toward accurate reconstruction of functional protein networks. Mol Syst Biol 2009; 5:248; http://dx.doi.org/10.1038/msb.2009.3; PMID: 19293828
- Beck T, Hall MN. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 1999; 402:689 - 92; http://dx.doi.org/10.1038/45287; PMID: 10604478
- Cunningham TS, Andhare R, Cooper TG. Nitrogen catabolite repression of DAL80 expression depends on the relative levels of Gat1p and Ure2p production in Saccharomyces cerevisiae. J Biol Chem 2000; 275:14408 - 14; http://dx.doi.org/10.1074/jbc.275.19.14408; PMID: 10799523
- Georis I, Tate JJ, Cooper TG, Dubois E. Nitrogen-responsive regulation of GATA protein family activators Gln3 and Gat1 occurs by two distinct pathways, one inhibited by rapamycin and the other by methionine sulfoximine. J Biol Chem 2011; 286:44897 - 912; http://dx.doi.org/10.1074/jbc.M111.290577; PMID: 22039046
- Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol 2003; 4:117 - 26; http://dx.doi.org/10.1038/nrm1018; PMID: 12563289
- Kulkarni A, Buford TD, Rai R, Cooper TG. Differing responses of Gat1 and Gln3 phosphorylation and localization to rapamycin and methionine sulfoximine treatment in Saccharomyces cerevisiae. FEMS Yeast Res 2006; 6:218 - 29; http://dx.doi.org/10.1111/j.1567-1364.2006.00031.x; PMID: 16487345
- Georis I, Tate JJ, Cooper TG, Dubois E. Tor pathway control of the nitrogen-responsive DAL5 gene bifurcates at the level of Gln3 and Gat1 regulation in Saccharomyces cerevisiae. J Biol Chem 2008; 283:8919 - 29; http://dx.doi.org/10.1074/jbc.M708811200; PMID: 18245087
- Schonbrun M, Laor D, López-Maury L, Bähler J, Kupiec M, Weisman R. TOR complex 2 controls gene silencing, telomere length maintenance, and survival under DNA-damaging conditions. Mol Cell Biol 2009; 29:4584 - 94; http://dx.doi.org/10.1128/MCB.01879-08; PMID: 19546237
- Weisman R, Choder M. The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J Biol Chem 2001; 276:7027 - 32; http://dx.doi.org/10.1074/jbc.M010446200; PMID: 11096119
- Kawai M, Nakashima A, Ueno M, Ushimaru T, Aiba K, Doi H, et al. Fission yeast tor1 functions in response to various stresses including nitrogen starvation, high osmolarity, and high temperature. Curr Genet 2001; 39:166 - 74; http://dx.doi.org/10.1007/s002940100198; PMID: 11409178
- Choo AY, Blenis J. TORgeting oncogene addiction for cancer therapy. Cancer Cell 2006; 9:77 - 9; http://dx.doi.org/10.1016/j.ccr.2006.01.021; PMID: 16473275
- Kaeberlein M, Powers RW 3rd, Steffen KK, Westman EA, Hu D, Dang N, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 2005; 310:1193 - 6; http://dx.doi.org/10.1126/science.1115535; PMID: 16293764
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 2003; 426:620; http://dx.doi.org/10.1038/426620a; PMID: 14668850
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 2009; 326:140 - 4; http://dx.doi.org/10.1126/science.1177221; PMID: 19797661
- Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A 1994; 91:9857 - 60; http://dx.doi.org/10.1073/pnas.91.21.9857; PMID: 7937905
- Nakashima A, Sato T, Tamanoi F. Fission yeast TORC1 regulates phosphorylation of ribosomal S6 proteins in response to nutrients and its activity is inhibited by rapamycin. J Cell Sci 2010; 123:777 - 86; http://dx.doi.org/10.1242/jcs.060319; PMID: 20144990