Abstract
It has been recently proposed that AMP-activated protein kinase (AMPK) might indirectly promote the phosphorylation of MRLC (myosin II regulatory light chain) at Ser19 to regulate the transition from metaphase to anaphase and the completion of cytokinesis. Although these findings provide biochemical support for our earlier observations showing that the active form of the α catalytic AMPK subunit associates dynamically with essential mitotic regulators, several important issues remained unexplored. Does glucose starvation alter the ability of AMPK to bind to the mitotic apparatus and travel from centrosomes to the spindle midzone during mitosis and cytokinesis? Does AMPK activate MRLC exclusively at the cleavage furrow during cytokinesis? What is the mitosis-specific stimulus that activates the mito-cytokinetic AMPK/MRLC axis regardless of energy deprivation? First, we confirm that exogenous glucose deprivation fails to alter the previously described distribution of phospho-AMPKαThr172 in all of the mitotic phases and does not disrupt its apparent association with the mitotic spindle and other structures involved in cell division. Second, we establish for the first time that phospho-AMPKαThr172 colocalizes exclusively with Ser19-phosphorylated MRLC at the cleavage furrow of dividing cells, a previously unvisualized interaction between phospho-AMPKαThr172 and phospho-MRLCSer19 that occurs in cleavage furrows, intercellular bridges and the midbody during cell division that appears to occur irrespective of glucose availability. Third, we reveal for the first time that the inhibition of AMPK mitotic activity in response to PLK1 inhibition completely prevents the co-localization of phospho-AMPKαThr172 and phospho-MRLCSer19 during the final stages of cytokinesis and midbody ring formation. Because PLK1 inhibition efficiently suppresses the AMPK-mediated activation of MRLC at the cytokinetic cleavage furrow, we propose a previously unrecognized role for AMPK in ensuring that cytokinesis occurs at the proper place and time by establishing a molecular dialog between PLK1 and MRLC in an energy-independent manner.
Keywords: :
A recent pioneering chemical genetic screen aimed at identifying direct substrates of a catalytic subunit of the energy-sensing AMP-activated protein kinase (AMPK) AMPKα2 revealed that the AMPK substrates PPP1R12C (phosphatase 1 regulatory subunit 12C) and PAK2 (p21-activated protein kinase) are directly involved in mitosis completion and chromosome segregation.Citation1 The authors proposed a model in which mitotic AMPK might indirectly promote the phosphorylation of MRLC (myosin II regulatory light chain) at Ser19 by inhibiting the phosphatase activity of PPP1R12C-PP1Cβ complexes and simultaneously acting through as-yet-unknown mechanisms involving PAK2.Citation1,Citation2 The observations by Banko et al. provide biochemical support for our earlier observations showing that the active form of the α-catalytic AMPK subunit associates dynamically with essential mitotic regulators, such as the centrosomal Aurora A kinase and the chromosomal passenger proteins (CPPs) Aurora B and INCENP at several mitosis-specific structures (e.g., centrosomes, spindle poles, the central spindle midzone and the cytokinetic midbody) throughout all phases of mitosis and during the furrowing process of cytokinesis.Citation3-Citation5 The findings of Banko et al. also confirm that mitotic progression is equally significantly impaired in response to either the inhibition or the activation of AMPK,Citation5,Citation6 leading to multinucleated cellular states. Therefore, the loss or activation of mitotic AMPK phenocopies the mitotic defects that are observed in response to the inhibition or activation of well-recognized mitotic apparatus-bound proteins.
Because the phosphorylation of Ser19 in MRLC is crucial to ensure the normal recruitment of the actin/myosin filaments that constrict the cell membrane to form a cleavage furrow,Citation7 Banko et al. proposed that the AMPK-driven activation of MRLC could regulate the transition from metaphase to anaphase and the completion of cytokinesis.Citation1 Moreover, glucose starvation failed to alter mitotic progression and pharmacological and genetic approaches aimed at specifically perturbing AMPK activity led to a significant increase in the number of multinucleated cells. Thus, the authors proposed that AMPK could regulate MRLC-dependent mitosis completion independently of low cellular energy status. As astutely suggested by Robitaille and Hall,Citation2 the findings reported by Banko et al. raise several important issues that remained, perhaps surprisingly, unexplored. Does glucose starvation alter the ability of AMPK to bind to the mitotic apparatus and travel from centrosomes to the spindle midzone during mitosis and cytokinesis? Does AMPK activate MRLC exclusively at the cleavage furrow during cytokinesis? What is the mitosis-specific stimulus that activates the mito-cytokinetic AMPK/MRLC axis regardless of energy deprivation? First, we showed that exogenous glucose deprivation fails to alter the previously described distribution of phospho-AMPKαThr172 in all of the mitotic phases and does not disrupt its apparent association with the mitotic spindle and other structures involved in cell division (). Second, we confirmed that Ser19-phosphorylated MRLC localizes to the cleavage furrow of dividing cells, suggesting that the phosphorylation of MRLC plays an important role in cytokinesis. More importantly, when examining the possibility that AMPK could phosphorylate MRLC during cytokinesis, we established for the first time that phospho-AMPKαThr172 colocalizes exclusively with Ser19-phosphorylated MRLC at the cleavage furrow of dividing cells (). Moreover, the previously unvisualized interaction between phospho-AMPKαThr172 and phospho-MRLCSer19 that occurs in cleavage furrows, intercellular bridges and the midbody during cell division appears to occur irrespective of glucose availability (). To confirm that mitosis-specific activators permit mitotic apparatus-bound AMPK to operate independently of low cellular energy status, we took advantage of the recently discovered link between polo-like kinase 1 (PLK1) activation and the mitotic phosphorylation of AMPKα.Citation8 Once cells have entered mitosis, the multi-faceted kinase PLK1 directs centrosome maturation and spindle formation, regulates the assembly of the bipolar spindle by promoting chromosome attachments to spindle microtubules, ensures the operation of the spindle checkpoint, including monitoring sister chromatid separation, and enables daughter cells to exit from mitosis by activating the anaphase-promoting complex/cyclosome (APC/C) and initiating cytokinesis.Citation9 Importantly, PLK1 and phospho-AMPKαThr172 exhibit a major spatio-temporal overlap at centrosomes from prophase until anaphase and at the midbody during telophase and cytokinesis.Citation8 Short-term treatment (30 min) with GW843682X (compound 1), a thiophene benzimidazole ATP-competitive inhibitor of PLK1, was sufficient to largely prevent the localization of phospho-AMPKαThr172 to several mitotic and cytokinetic structures, and this inhibitory effect occurred independently of glucose availability. More importantly, the inhibition of AMPK mitotic activity in response to PLK1 inhibition completely prevented the co-localization of phospho-AMPKαThr172 and phospho-MRLCSer19 during the final stages of cytokinesis and midbody ring formation (). Because the phosphorylation of Ser19 of MRLC stimulates the actin-activated ATPase activity of myosin, which is important for the proper localization of myosin to the furrow and for ingression,Citation10 it is reasonable to suggest that PLK1 might direct the AMPK activation of MRLC to allow for the ingression of the cleavage furrow to continue until the midbody structure is formed. Indeed, stabilization phenomena, in which chromosomes appear to produce a thin cytoplasmatic bridge of chromatin uniting the two daughter cells, can be observed upon the loss of MRLC Ser19 phosphorylation that occurs in response to the inactivation of the PLK1/AMPK axis ().
Figure 1. Spatio-temporal dynamics of phospho-AMPKαThr172 during mitosis and cytokinesis in glucose-starved A431 epidermoid cancer cells. Figure shows representative portions of glucose-starved (18 h), cell dividing-containing images captured on a BD PathwayTM 855 Bioimager System with a 40x objective in different channels for fosfo-AMPKαThr172 (green) and Hoechst 33258 (blue) and merged using BD AttovisionTM software. A rabbit anti-phospho-AMPK (Thr172) polyclonal antibody (sc-101630, Santa Cruz Biotechnology) was used in this set of images (and also in –)
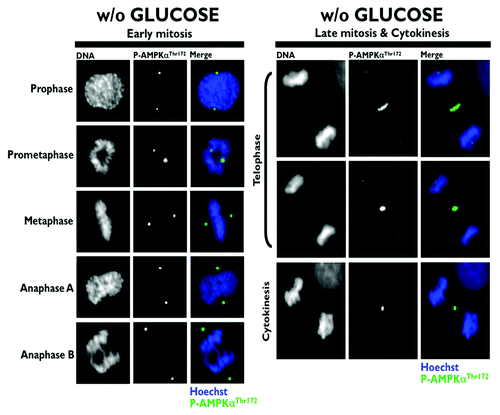
Figure 2. Co-localization analysis of phospho-AMPKαThr172 and phospho-MRLCSer19 during mitosis and cytokinesis in glucose-supplemented A431 epidermoid cancer cells. Figure shows representative portions of cell dividing-containing images captured on a BD PathwayTM 855 Bioimager System with a 40x objective in different channels for phospho-AMPKThr172 (red), phospho-MRCLSer19 (green) and Hoechst 33258 (blue) and merged using BD AttovisionTM software. The rectangular regions (white line) are enlarged and shown as high magnification insets. A phospho-myosin light chain 2 (Ser19) mouse monoclonal antibody (#3675) was used in the set of images (and also in and ).
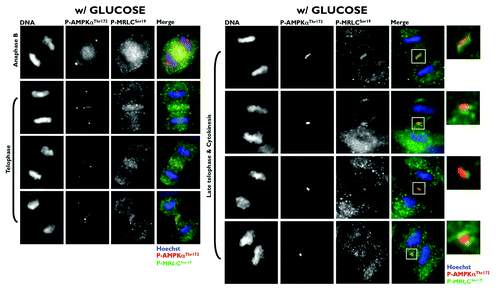
Figure 3. Co-localization analysis of phospho-AMPKαThr172 and phospho-MRLCSer19 during mitosis and cytokinesis in glucose-starved A431 epidermoid cancer cells. Figure shows representative portions of glucose-starved (18 h), cell dividing-containing images captured on a BD PathwayTM 855 Bioimager System with a 40x objective in different channels for phospho-AMPKThr172 (red), phospho-MRCLSer19 (green) and Hoechst 33258 (blue) and merged using BD AttovisionTM software. The rectangular regions (white line) are enlarged and shown as high magnification insets.
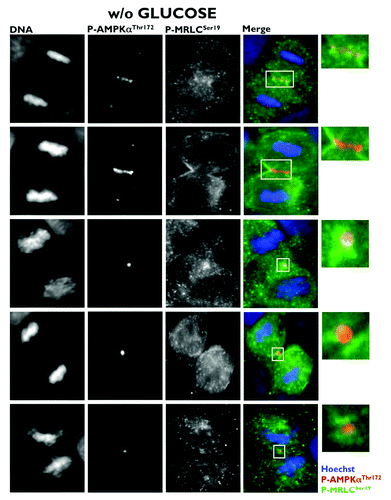
Figure 4. Spatio-temporal dynamics of phospho-AMPKαThr172 and phospho-MRLCSer19 during mitosis and cytokinesis: Impact of the PLK1 inhibitor GW843682X. Glucose-supplemented (left panels) and glucose-starved (right panels) A431 cells were treated with 10 μmol/L GW843682X for 30 min. Figure shows representative portions of cell dividing-containing images captured on a BD PathwayTM 855 Bioimager System with a 40x objective in different channels for phospho-AMPKThr172 (red), phospho-MRCLSer19 (green) and Hoechst 33258 (blue) and merged using BD AttovisionTM software. The rectangular regions (white line) are enlarged and shown as high magnification insets.
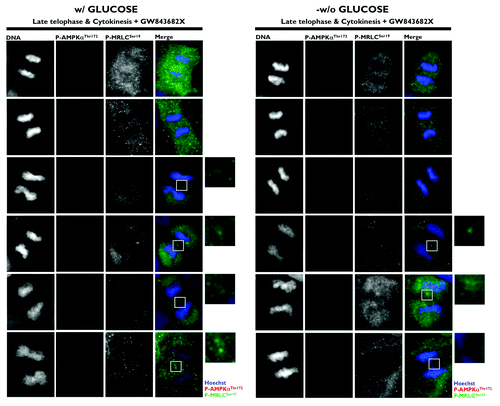
PLK1 is a well-recognized positive regulator of cytokinesis in mammalian cells that localizes at the midbody during telophase and cytokinesis. Surprisingly, active Thr172-phorphorylated AMPKα and Ser19-phosphorylated MRCL both localize at the midbody.Citation3-Citation5 Because PLK1 inhibition efficiently suppresses the AMPK-mediated activation of MRLC at the cytokinetic cleavage furrow, we propose a new role for AMPK in ensuring that cytokinesis occurs at the proper place and time by establishing a molecular dialog between PLK1 and MRLC in an energy-independent manner.
Acknowledgments
This work was financially supported through funding from the Instituto de Salud Carlos III [Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria (FIS), Grants CP05–00090 and PI06–0778 and RD06–0020–0028], the Fundación Científica de la Asociación Española Contra el Cáncer (AECC) and the Ministerio de Ciencia e Innovación (SAF2009–11579, Plan Nacional de I+D+ I, MICINN). Alejandro Vazquez-Martin received the Sara Borrell post-doctoral contract (CD08/00283, Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria–FIS). Sílvia Cufí received a Research Fellowship (Formación de Personal Investigador, FPI) from the Ministerio de Ciencia e Innovación (MICINN).
References
- Banko MR, Allen JJ, Schaffer BE, Wilker EW, Tsou P, White JL, et al. Chemical genetic screen for AMPKα2 substrates uncovers a network of proteins involved in mitosis. Mol Cell 2011; 44:878 - 92; http://dx.doi.org/10.1016/j.molcel.2011.11.005; PMID: 22137581
- Robitaille AM, Hall MN. Ramping up mitosis: an AMPKα2-regulated signaling network promotes mitotic progression. Mol Cell 2012; 45:8 - 9; http://dx.doi.org/10.1016/j.molcel.2011.12.018; PMID: 22244327
- Vazquez-Martin A, López-Bonet E, Oliveras-Ferraros C, Pérez-Martínez MC, Bernadó L, Menendez JA. Mitotic kinase dynamics of the active form of AMPK (phospho-AMPKalphaThr172) in human cancer cells. Cell Cycle 2009; 8:788 - 91; http://dx.doi.org/10.4161/cc.8.5.7787; PMID: 19221486
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The active form of the metabolic sensor: AMP-activated protein kinase (AMPK) directly binds the mitotic apparatus and travels from centrosomes to the spindle midzone during mitosis and cytokinesis. Cell Cycle 2009; 8:2385 - 98; http://dx.doi.org/10.4161/cc.8.15.9082; PMID: 19556893
- Vazquez-Martin A, Oliveras-Ferraros C, Lopez-Bonet E, Menendez JA. AMPK: Evidence for an energy-sensing cytokinetic tumor suppressor. Cell Cycle 2009; 8:3679 - 83; http://dx.doi.org/10.4161/cc.8.22.9905; PMID: 19844168
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 2007; 447:1017 - 20; http://dx.doi.org/10.1038/nature05828; PMID: 17486097
- Asano S, Hamao K, Hosoya H. Direct evidence for roles of phosphorylated regulatory light chain of myosin II in furrow ingression during cytokinesis in HeLa cells. Genes Cells 2009; 14:555 - 68; http://dx.doi.org/10.1111/j.1365-2443.2009.01288.x; PMID: 19371382
- Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Menendez JA. Polo-like kinase 1 regulates activation of AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell Cycle 2011; 10:1295 - 302; http://dx.doi.org/10.4161/cc.10.8.15342; PMID: 21474997
- Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov 2010; 9:643 - 60; http://dx.doi.org/10.1038/nrd3184; PMID: 20671765
- Normand G, King RW. Understanding cytokinesis failure. Adv Exp Med Biol 2010; 676:27 - 55; http://dx.doi.org/10.1007/978-1-4419-6199-0_3; PMID: 20687468