Abstract
Signaling pathways orchestrated by PI3K/Akt, Raf/Mek/Erk and Wnt/β-catenin are known to play key roles in the self-renewal and differentiation of pluripotent stem cells. The serine/threonine protein kinase Gsk3β has roles in all three pathways, making its exact function difficult to decipher. Consequently, conflicting reports have implicated Gsk3β in promoting self-renewal, while others suggest that it performs roles in the activation of differentiation pathways. Different thresholds of Gsk3β activity also have different biological effects on pluripotent cells, making this situation even more complex. Here, we describe a further level of complexity that is most apparent when comparing “naïve” murine and “primed” human pluripotent stem cells. In naïve cells, Gsk3β activity is restrained by PI3K/Akt, but when released from inhibitory signals it antagonizes self-renewal pathways by targeting pluripotency factors such as Myc and Nanog. This situation also applies in primed cells, but, in addition, a separate pool of Gsk3β is required to suppress canonical Wnt signaling. These observations suggest that different Gsk3β-protein complexes shift the balance between naïve and primed pluripotent cells and identify fundamental differences in their cell signaling. Altogether, these findings have important implications for the mechanisms underpinning the establishment of different pluripotent cell states and for the control of self-renewal and differentiation.
Introduction
Self-renewal of pluripotent stem cells is dependent on the maintenance of proliferation and the suppression of differentiation pathways. We recently described how a crosstalk mechanism between PI3K/Akt, Raf/Mek/Erk, Activin A/Smad2,3 and Wnt/β-catenin signaling networks maintains pluripotency of human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs).Citation1 This signaling network seems to be characteristic of “primed” pluripotent stem cells (PSCs). In “naïve” murine ESCs (mESCs), however, self-renewal is dependent on the activation of PI3K/Akt,Citation2 Lif/Stat3 and the BMP/SmadCitation3 pathway. Together, these pathways culminate in the inhibition of Erk and Gsk3β activity.Citation4 Importantly, the only common pathway required by murine and human PSCs involves signaling through PI3K/Akt.
The downstream targets of Akt in murine and human PSCs have been described in detail. In murine cells, Akt directly phosphorylates Gsk3β on serine-9 (S9), keeping it inactiveCitation5-Citation7 while also regulating its ability to shuttle in and out of the nucleus and affecting its ability to target substrates such as c-myc.Citation8,Citation9 Although inhibition of PI3K/Akt signaling in human and murine PSCs results in differentiation,Citation10,Citation11 its function is subtly different in the naïve and primed states. PI3K/Akt has two roles in primed human PSCs. First, it establishes levels of Activin A/Smad2,3, signaling that is compatible with expression of pluripotency genes such as Nanog. Under self-renewal conditions, the magnitude of Smad2,3 activity is not sufficient for activation of early differentiation genes, such as MixL1,Citation1 but is nevertheless sufficient for the maintenance of pluripotency. In its second role, PI3K/Akt maintains the activity of a separate pool of Gsk3 involving a crosstalk mechanism requiring inhibition of Raf/Mek/Erk signaling.Citation1 This second pool of Gsk3 serves to antagonize canonical Wnt/β-catenin signaling. These studies establish multiple roles for Gsk3 in pluripotent cells that could account for some of the major differences between the primed and naïve states.
Major differences in Gsk3 function and regulation were clearly not anticipated at the beginning of these studies. To reconcile the differences between human and murine PSCs, we set out to test the general hypothesis that differences in Gsk3β activity could be a feature of distinct pluripotent cell states. Our studies indicate that Gsk3β is negatively targeted in murine PSCs by PI3K/Akt signaling, but, once activated, it antagonizes c-myc activity. In human PSCs, this situation also applies, but, additionally, a separate pool of active Gsk3β is required to suppress canonical Wnt signaling. This does not appear to be a requirement in naïve PSCs, however. These data provide a framework to explain differences in signaling requirements of naïve and primed pluripotent stem cells and identify key biochemical differences between the two pluripotent cell states.
Results
Different modes of Gsk3β regulation in human and murine pluripotent stem cells.
Inhibition of Gsk3β, along with Erk inhibition, has been shown to support the self-renewal and pluripotency of mESCs.Citation4 Human PSCs, however, have been shown to undergo differentiation under conditions where Gsk3β is strongly inhibited.Citation1 To investigate these seemingly contradictory findings, we directly compared the subcellular localization and phosphorylation status of Gsk3β in human PSCs grown in HAI/StemPro mediaCitation1 or murine PSCs grown in containing leukemia inhibitory factor (LIF) and fetal calf serum (FCS)Citation5 (). In both human and murine PSCs, Gsk3β is localized to the cytoplasm, consistent with our previous reports describing the mechanisms of Gsk3β shuttling in and out of the nucleus.Citation8,Citation9 The phosphorylation status of Gsk3β on S9 was considerably different, however, being predominantly hyper-phosphorylated in murine PSCs and hypo-phosphorylated in human PSCs (). As S9 phosphorylation on Gsk3β is indicative of inactivation,Citation5-Citation7 this suggests very different modes of Gsk3β regulation in mouse and human PSCs. In both murine and human PSCs, Nanog is robustly expressed, and β-catenin is primarily associated with the plasma membrane.
Figure 1. Gsk3 phosphorylation status differs in human and mouse pluripotent stem cell maintenance and differentiation. (A) Immunostaining for Gsk3β, pGsk3βS9, Nanog, and β-catenin during mouse or human pluripotent stem cell maintenance. (B) IP-kinase assays of Gsk3β following treatment with BIO (2 μM) for 24 h. (C) Immunoblotting for human and murine ESCs or human and murine EBs following aggregation in serum for 48 h with indicated antibodies. (D) qRT-PCR analysis of mESCs and embryoid bodies (48 h). Assays were performed in triplicate and normalized against a Gapdh control. Data are expressed +/− SEM (E) Model showing changes in global Gsk3 activity as pluripotent cells transition between naïve, primed and committed states.
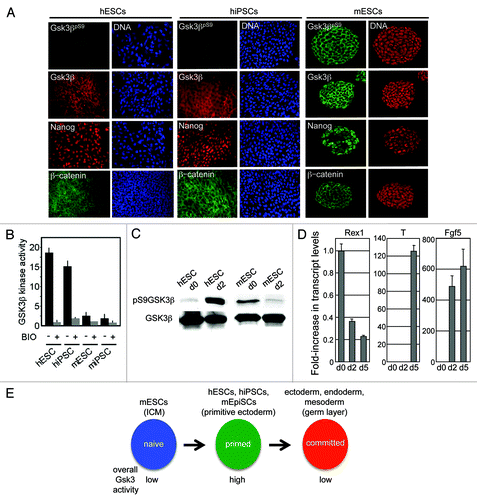
To more directly evaluate Gsk3β activity, we performed immunoprecipitation-kinase assays from whole-cell lysates (). As anticipated from previous phospho-S9 analysis, human PSCs exhibit high steady-state Gsk3β kinase activity. This activity is suppressed by pre-treatment of cells with the Gsk3 inhibitor, 2'Z,3'E)-6-Bromoindirubin-3'-oxime (BIO). In contrast, murine PSCs show a comparatively low level of kinase activity, consistent with the requirement to suppress Gsk3β for maintenance of naïve mESCs.Citation4 The comparatively low level of Gsk3 activity in murine PSCs is also sensitive to BIO treatment (). These data confirm our earlier findings that global Gsk3β activity is low in murine PSCs and high in human PSCs.
We next examined Gsk3β activity during the early stages of differentiation using S9 phosphorylation as a read-out (). Embryoid bodies (EBs) were formed by aggregation of mouse or human PSCs in FCS-containing media to induce differentiation. In hESCs we found an increase in S9 phosphorylation within 2 d of EB formation, while in mESCs, S9 phosphorylation decreased within this time period. These differences were initially surprising, but we reasoned that after 2 d differentiation, murine PSCs would be transitioning through a Rex1- Fgf5+ Nanog+ Oct4+ pluripotent, primitive ectoderm state before becoming lineage committed. This was confirmed by qRT-PCR (). These data indicate that as murine PSCs transition from a naïve to primed state, global Gsk3 activity increases (). These observations reconcile the differences between mESCs and hESCs and indicate that elevated Gsk3 activity is a hallmark of primed PSCs.
Gsk3 suppresses canonical Wnt activity in human PSCs but not murine PSCs.
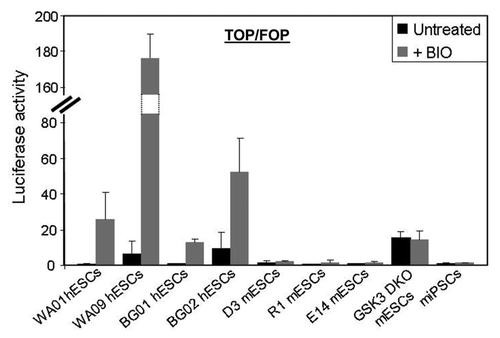
Evaluation of Wnt signaling with the Top-Flash luciferase reporter assay revealed several key features of β-catenin signaling in human and murine PSCs. First, Top-Flash activity in human PSCs (WA01, WA09, BG01 and BG02) is low but activated by addition of BIO (), indicating that Wnt signaling in human pluripotent cells is suppressed due to elevated Gsk3β activity. Although the trends are similar, there are clear differences in the ability of different human PSC lines to upregulate Wnt reporter activity in response to BIO. This may explain variations in self-renewal and differentiation properties typically seen in different pluripotent cell lines. All murine PSCs assayed (D3, R1, E14 and miPSCs) showed low, BIO-insensitive Wnt reporter activity. The only exception to this was a murine Gsk3α/β-knockout mESC line that exhibited moderate levels of BIO-insensitive Top-Flash activity (). These findings indicate that Gsk3β activity is critical for restraining canonical Wnt signaling in human PSCs but not in murine PSCs. The inability to activate β-catenin-dependent transcription by inhibition of Gsk3 in murine PSCs indicates that the canonical Wnt pathway has not been established in naïve cells. This is consistent with our earlier model,Citation1 where Wnt signaling is actively suppressed in human pluripotent cells due to elevated Gsk3β activity (see Discussion).
Different roles for PI3K in regulation of Gsk3 activity in human and murine PSCs.
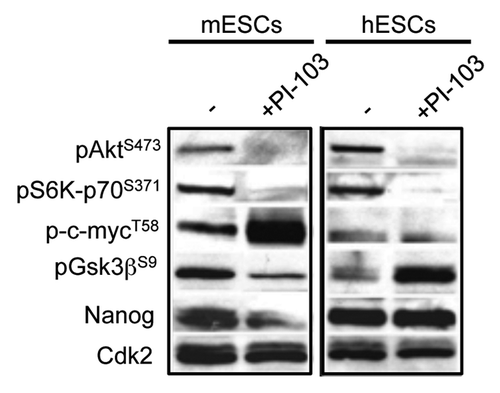
In mESCs, inhibition of PI3K/Akt signaling results in Gsk3β activation.Citation5-Citation9 In human PSCs, however, this results in Erk activation and Gsk3β inactivation.Citation1 Given these opposite findings, we decided to directly compare the response to PI3K/Akt inhibition in mouse and human PSCs (). Upon treatment of human and mouse PSCs with the PI3K inhibitor, PI-103, phosphorylation of Akt on serine-473 (S473) and phosphorylation on the Akt substrate, p70-S6 kinase on serine-371 (S371) both decreased. This confirms that under these conditions, PI3K signaling is blocked. Interestingly, the phosphorylation status of Gsk3β was considerably different in mouse and human ESCs after PI3K inhibition. In mESCs, Gsk3β S9 phosphorylation decreased indicating increased activity, while in hESCs, S9 phosphorylation increased, indicative of decreased activity. The Gsk3β target, c-myc, showed increased phosphorylation on threonine-58 (T58) in mESCs upon PI3K inhibition, but no significant response in hESCs. Altogether, these data show that upon the loss of PI3K/Akt signaling, global Gsk3β activity and its targets respond differently in human and mouse PSCs. Other downstream effectors of PI3K signaling, such as Akt and S6K, respond in a similar manner. The significance of this is described in detail below.
Discussion
PI3K/Akt signaling is essential to the self-renewal of murine and human pluripotent stem cells. In mPSCs, one of the primary functions for PI3K/Akt is to suppress Gsk3β activity by maintaining its hyper-phosphorylation on S9.Citation5-Citation7 This establishes high levels of Myc, which are essential to maintain self-renewal and pluripotency through several mechanisms, including cell cycle control,Citation13 repressing primitive endoderm formation,Citation14 blocking Erk signalingCitation15 and regulating transcriptional pause-release.Citation16 Myc has also been shown to be important for reprogramming.Citation17,Citation18
Human PSCs also require high levels of PI3K/Akt signaling to promote self-renewal.Citation1,Citation10,Citation19 However, a major function of Akt in human pluripotent stem cells is to suppress Erk signaling, which maintains a separate pool of Gsk3β in an active, hypo-phosphorylated state.Citation1 High levels of active Gsk3β suppress Wnt/β-catenin signaling to promote pluripotency. Altogether, this establishes a scenario where Gsk3β exists in at least two different networks, one that is part of the canonical PI3K pathway and functions by targeting pluripotency regulators, such as Nanog and c-myc. The second network involves a pool of Gsk3 under the control of Erk that impacts on the canonical Wnt pathway. The second pathway doesn’t appear to be intact in naïve cells and seems to be acquired as naïve PSCs transition to a primed state (). The evidence for this model is most apparent when examining downstream targets of Gsk3β in different stem cell types. For example, inhibition of Gsk3β only weakly activated the β-catenin reporter in mouse stem cells but strongly activated it in human stem cells. Additionally, inhibition of PI3K considerably changed Gsk3β activity based on S9 phosphorylation in mouse and human stem cells but only affected c-myc phosphorylation in mouse stem cells.
Figure 4. Gsk3β exists in different regulatory networks in pluripotent stem cells. Our findings suggest that in mPSCs, Gsk3β activity is primarily linked to the canonical PI3K pathway and targets substrates such a Nanog and c-myc. In hPSCs, Gsk3β forms complexes with ErkCitation1 and is directed toward the canonical Wnt pathway and members of the “β-catenin destruction complex.” These data suggest that Gsk3β is recruited into additional signaling pathways as naïve cells transition to primed PSCs.
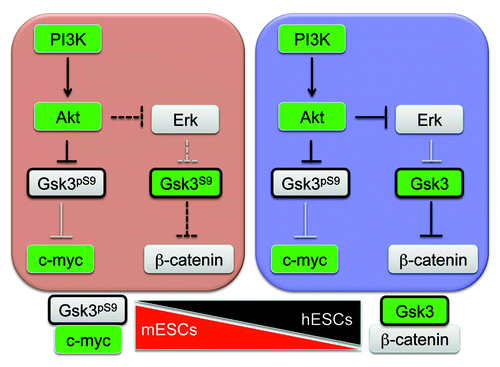
Mouse and human pluripotent stem cells are not equivalent stem cell states. Human pluripotent stem cells have been found to be most similar to mouse epiblast stem cells (EpiSCs),Citation20,Citation21 which are from a later stage in development than mouse ESCs. This indicates that during embryonic development there are different levels of pluripotency. Mouse ESCs are considered to represent naïve or early-stage pluripotent stem cells, while human ESCs and mouse EpiSCs represent primed or late-stage pluripotent stem cells. Our findings here suggest that during maturation of pluripotent cells from a naïve to a primed state, Gsk3β shifts from Akt-Myc complexes to Wnt/β-catenin-Erk complexes. High levels of Gsk3β activity found in hESCs suggests a fully functioning “β-catenin destruction complex,” which typically consists of Axin, APC, β-catenin, Gsk3β, CK1 and PP2A, that plays a direct role in regulating Wnt signaling.Citation22 A shift in complexes by Gsk3β therefore permits tightly controlled regulation of Wnt/β-catenin signaling needed during embryonic development. The high level of responsiveness by the β-catenin reporter from BIO treatment in hESCs, but not mESCs, further indicates that these cells are now “primed” for Wnt signaling to stimulate differentiation. Different hESC lines also vary in their degree of β-catenin activation, in response to BIO. This explains common differences found in differentiation potential between different pluripotent cell lines.
While Wnt/β-catenin signaling clearly promotes differentiation in hPSCs, its role in mPSCs has been less clear. Recent work has found that β-catenin is not required for mouse pluripotent stem cell self-renewal.Citation23,Citation24 However, it may still support pluripotency by regulating genes independently of β-catenin activation of target genes through a repression mechanism involving Tcf1 and Tcf3.Citation25 In agreement with this, recent work suggests that Wnt signaling favors naïve pluripotency (mouse ESCs) at the expense of primed pluripotency (EpiSCs).Citation12
In sum, we have described differences in the abundance of Gsk3β in distinct protein complexes existing in mouse and human pluripotent stem cells. These differences account for opposite effects of Gsk3 inhibitors in murine and human stem cells and expose what could be a major determinant of the naïve vs. primed pluripotent state. Altogether, these findings have important implications for signaling mechanisms in the different pluripotent states found during early embryonic development.
Materials and Methods
Cell culture.
hESCs (WA01 and WA09, from WiCell and BG01, BG02 from ViaCyte) and hiPSCs (Fib2-iPS4, from Dr. George Daley) were maintained in HAI (basis for StemPro® hESC SFM, Invitrogen), by a variation to the method previously described.Citation1,Citation26 Recombinant factors used in defined media were as follows; heregulin (HFG1β, 10 ng/ml, Peprotech), Activin A (10 ng/ml, R&D Systems), Igf-1 (LrCitation3-Igf1, 200 ng/ml, JRH Biosciences). R1, E14, Gsk3 DKO mESCs and miPSCsCitation8 were maintained in +LIF complete media as previously described.Citation5 Inhibitors, 2'Z,3'E)-6-Bromoindirubin-3'-oxime, BIO/GSK3 inhibitor IX (Calbiochem) and PI-103 (Calbiochem) were used as described.
Immunostaining and immunoblotting.
Immunostaining was performed as follows: cells were fixed in 4% paraformaldehyde and blocked in 10% donkey serum in phosphate-buffered saline with 0.02% Triton X-100 (PBST) for 1 h. Cells were incubated with the indicated antibody overnight in blocking solution. Cells were washed with PBST and incubated with fluorescently labeled secondary antibodies for 1 h in blocking solution. Cells were washed in PBST and treated with mounting medium. Immunoblotting was performed as follows: cells were lysed in RIPA buffer for 30 min on ice with phosphatase and protease inhibitors. Following centrifugation to remove cell debris, lysate was mixed with an equal volume of 2X Laemmli sample buffer. Approximately 20 μg of protein was separated by SDS-PAGE and blotted on nitrocellulose membranes. Blots were blocked for 1 h in 4% non-fat dried milk in Tris-buffered saline with 0.1% Tween-20 (TBST) and then incubated with the indicated antibodies overnight in blocking in blocking solution. Blots were washed in TBST and then incubated with HRP-conjugated secondary antibodies in blocking solution for 1 h. Blots were washed again in TBST, and proteins were detected using ECL western blotting detection reagents (GE Healthcare) according to manufacturer instructions. The following antibodies were used for immunoblotting and immunostaining, Gsk3β, pGsk3βS9, pAktS473, pS6K-p70S371, p-c-mycT58 (Cell Signaling Technologies, Nanog (ReproCell), Cdk2 (Santa Cruz Biotechnology), and β-catenin (BD Biosciences).
Kinase assays and luciferase assays.
Immunoprecipitation-kinase assays were performed as described previously.Citation5 Luciferase assays were performed using the Top-Flash and Fop-Flash luciferase reporters using the Dual-Luciferase Reporter Kit (Promega) according to manufacturer instructions and normalized to a Renilla luciferase control.
| Abbreviations: | ||
| hESC | = | human embryonic stem cells |
| mESC | = | murine embryonic stem cells |
| PSC | = | pluripotent stem cells |
| EpiSCs | = | epiblast stem cells |
| EBs | = | embryoid bodies |
| iPSC | = | induced pluripotent stem cell |
| LIF | = | leukemia inhibitory factor |
| FCS | = | fetal calf serum |
| BIO | = | 2′Z,3′E)-6-Bromoindirubin-3′-oxime |
Acknowledgments
This work was supported by grants to S.D. from the National Institute of Child Health and Human Development (RO1 HD049647) and the National Institute for General Medical Sciences (PO1 GM085354).
References
- Singh AM, Reynolds D, Cliff T, Ohtsuka S, Mattheyses AL, Sun Y, et al. Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell 2012; 10:312 - 26; http://dx.doi.org/10.1016/j.stem.2012.01.014; PMID: 22385658
- Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 2006; 25:2697 - 707; http://dx.doi.org/10.1038/sj.onc.1209307; PMID: 16407845
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 2003; 115:281 - 92; http://dx.doi.org/10.1016/S0092-8674(03)00847-X; PMID: 14636556
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, et al. The ground state of embryonic stem cell self-renewal. Nature 2008; 453:519 - 23; http://dx.doi.org/10.1038/nature06968; PMID: 18497825
- Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 2005; 132:885 - 96; http://dx.doi.org/10.1242/dev.01670; PMID: 15673569
- Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem 2004; 279:48063 - 70; http://dx.doi.org/10.1074/jbc.M406467200; PMID: 15328362
- Storm MP, Bone HK, Beck CG, Bourillot PY, Schreiber V, Damiano T, et al. Regulation of Nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J Biol Chem 2007; 282:6265 - 73; http://dx.doi.org/10.1074/jbc.M610906200; PMID: 17204467
- Bechard M, Dalton S. Subcellular localization of glycogen synthase kinase 3beta controls embryonic stem cell self-renewal. Mol Cell Biol 2009; 29:2092 - 104; http://dx.doi.org/10.1128/MCB.01405-08; PMID: 19223464
- Bechard M, Trost R, Singh AM, Dalton S. Frat is a phosphatidylinositol 3-kinase/Akt-regulated determinant of glycogen synthase kinase 3β subcellular localization in pluripotent cells. Mol Cell Biol 2012; 32:288 - 96; http://dx.doi.org/10.1128/MCB.05372-11; PMID: 22064483
- McLean AB, D’Amour KA, Jones KL, Krishnamoorthy M, Kulik MJ, Reynolds DM, et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells 2007; 25:29 - 38; http://dx.doi.org/10.1634/stemcells.2006-0219; PMID: 17204604
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 2004; 10:55 - 63; http://dx.doi.org/10.1038/nm979; PMID: 14702635
- ten Berge D, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, et al. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol 2011; 13:1070 - 5; http://dx.doi.org/10.1038/ncb2314; PMID: 21841791
- Singh AM, Dalton S. The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming. Cell Stem Cell 2009; 5:141 - 9; http://dx.doi.org/10.1016/j.stem.2009.07.003; PMID: 19664987
- Smith KN, Singh AM, Dalton S. Myc represses primitive endoderm differentiation in pluripotent stem cells. Cell Stem Cell 2010; 7:343 - 54; http://dx.doi.org/10.1016/j.stem.2010.06.023; PMID: 20804970
- Hishida T, Nozaki Y, Nakachi Y, Mizuno Y, Okazaki Y, Ema M, et al. Indefinite self-renewal of ESCs through Myc/Max transcriptional complex-independent mechanisms. Cell Stem Cell 2011; 9:37 - 49; http://dx.doi.org/10.1016/j.stem.2011.04.020; PMID: 21726832
- Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, et al. c-Myc regulates transcriptional pause release. Cell 2010; 141:432 - 45; http://dx.doi.org/10.1016/j.cell.2010.03.030; PMID: 20434984
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663 - 76; http://dx.doi.org/10.1016/j.cell.2006.07.024; PMID: 16904174
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861 - 72; http://dx.doi.org/10.1016/j.cell.2007.11.019; PMID: 18035408
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 2005; 23:1534 - 41; http://dx.doi.org/10.1038/nbt1163; PMID: 16258519
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007; 448:196 - 9; http://dx.doi.org/10.1038/nature05972; PMID: 17597760
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 2007; 448:191 - 5; http://dx.doi.org/10.1038/nature05950; PMID: 17597762
- Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene 2006; 25:7482 - 91; http://dx.doi.org/10.1038/sj.onc.1210055; PMID: 17143292
- Lyashenko N, Winter M, Migliorini D, Biechele T, Moon RT, Hartmann C. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat Cell Biol 2011; 13:753 - 61; http://dx.doi.org/10.1038/ncb2260; PMID: 21685890
- Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, et al. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol 2011; 13:838 - 45; http://dx.doi.org/10.1038/ncb2267; PMID: 21685889
- Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, et al. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol 2011; 13:762 - 70; http://dx.doi.org/10.1038/ncb2283; PMID: 21685894
- Wang L, Schulz TC, Sherrer ES, Dauphin DS, Shin S, Nelson AM, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood 2007; 110:4111 - 9; http://dx.doi.org/10.1182/blood-2007-03-082586; PMID: 17761519