Abstract
Our studies revealed that LCA (lithocholic bile acid) extends yeast chronological lifespan if added to growth medium at the time of cell inoculation. We also demonstrated that longevity in chronologically aging yeast is programmed by the level of metabolic capacity and organelle organization that they developed before entering a quiescent state and, thus, that chronological aging in yeast is likely to be the final step of a developmental program progressing through at least one checkpoint prior to entry into quiescence. Here, we investigate how LCA influences longevity and several longevity-defining cellular processes in chronologically aging yeast if added to growth medium at different periods of the lifespan. We found that LCA can extend longevity of yeast under CR (caloric restriction) conditions only if added at either of two lifespan periods. One of them includes logarithmic and diauxic growth phases, whereas the other period exists in early stationary phase. Our findings suggest a mechanism linking the ability of LCA to increase the lifespan of CR yeast only if added at either of the two periods to its differential effects on various longevity-defining processes. In this mechanism, LCA controls these processes at three checkpoints that exist in logarithmic/diauxic, post-diauxic and early stationary phases. We therefore hypothesize that a biomolecular longevity network progresses through a series of checkpoints, at each of which (1) genetic, dietary and pharmacological anti-aging interventions modulate a distinct set of longevity-defining processes comprising the network; and (2) checkpoint-specific master regulators monitor and govern the functional states of these processes.
Introduction
One way to look at the complexity of the aging process, in which a limited number of evolutionarily conserved nutrient- and energy-sensing signaling pathways (hereafter often called “master regulators”) orchestrate a plethora of cellular processes in space and time (for a review, see refs. Citation1–Citation4), is to consider each of these numerous processes as a functional module integrated with other modules into a biomolecular network.Citation5-Citation8 In this conceptual framework, (1) a synergistic action of individual modules comprising the network could define longevity by establishing the rate of cellular and organismal aging; and (2) the relative impact of each module on the rate of aging in a particular organism or cell type could differ at various stages of its lifetime and could also vary in different organisms and cell types.Citation5-Citation10 Initially developed for theoretical modeling of the complexity of the senescence process in mammalian organisms,Citation11 a network biology approach has been intensively used in recent years for reconstructing biomolecular networks of longevity and aging on the cellular and organismal levels. Numerous models of such networks have been proposed based on system biological and computational analyses of protein-protein interactions, age-related changes in gene expression or longevity-defining metabolic alterations.Citation4,Citation10,Citation12-Citation20
Here we extend the network theory of aging by proposing that a biomolecular longevity network could progress through a series of checkpoints. At each of these checkpoints, a distinct set of master regulators senses the functional states of critical modules comprising the network. Based on this information and considering the input of some environmental cues (such as caloric and dietary intake, environmental stresses, endocrine factors, etc.), master regulators modulate certain processes within monitored modules in order to limit the age-related accumulation of molecular and cellular damage. The resulting changes in the dynamics of individual modules comprising the network and in its general configuration are critically important for defining longevity by establishing the rate of cellular and organismal aging.
A confirmation of this concept for a stepwise development of a longevity network configuration at a series of checkpoints, each being monitored by a limited set of checkpoint-specific master regulators, comes from studies that revealed distinctive timing requirements for modulating the pace of organismal aging in the nematode Caenorhabditis elegans. It seems that this organism employs at least three independent regulatory systems that, by monitoring a particular cellular process or processes during a specific stage of life, use this information to establish the rate of cellular and organismal aging. The first of these three regulatory systems influences lifespan by operating only during larval development.Citation21,Citation22 By monitoring mitochondrial respiration, electrochemical membrane potential and ATP production early in life, during the L3/L4 larval stage, it establishes the rate of aging that persists during adulthood.Citation21,Citation22 Mechanisms underlying the essential role of this first regulatory system in defining longevity of C. elegans may involve (1) a remodeling of mitochondria-confined ATP production pathways during larval development, which may establish a specific configuration of the longevity-defining metabolic network in a cell-autonomous manner;Citation23,Citation24 and (2) a retrograde signaling pathway that, in response to mild mitochondrial impairment and stress during the L3/L4 larval stage, activates UBL-5 (ubiquitin-like protein 5)/DVE-1 (defective proventriculus protein 1)-driven expression of the mitochondria-specific unfolded protein response (UPRmt) genes in the nucleus, thereby stimulating synthesis of a subset of UPRmt proteins that can extend longevity, not only cell-autonomously, but also in a cell-non-autonomous fashion.Citation25 The second regulatory system operates exclusively during adulthood, mainly during early adulthood, to influence the lifespan of C. elegans via the insulin/IGF-1 (insulin-like growth factor 1) longevity signaling pathway and the transcription factor DAF-16 (dauer formation protein 16).Citation26,Citation27 Noteworthy, the magnitude of the effect of this second regulatory system on lifespan declines with age, becoming insignificant after several days of adulthood.Citation26 The third regulatory system influences the lifespan of C. elegans in a diet-restriction-specific fashion by operating exclusively during adulthood.Citation28 This system regulates longevity via the transcription factor PHA-4 (pharynx development protein 4) only in response to reduced food intake. Importantly, the PHA-4-mediated regulatory system operates independently of the other two regulatory systems modulating the rate of aging in C. elegans.Citation28 In our hypothesis for a stepwise development of a longevity network configuration at a series of checkpoints, a genetic, dietary or pharmacological anti-aging intervention may modulate the key cellular process or processes that are monitored at a particular checkpoint by a master regulator of the longevity control system. In C. elegans, UBL-5/DVE-1, DAF-16 and PHA-4 may function as the checkpoint-specific master regulators of longevity by governing progression through the three consecutive checkpoints operating during larval development and early adulthood.Citation25-Citation28
It seems that some general aspects of the hypothesis proposed here of the longevity control system progressing through a series of checkpoints could be applicable to laboratory mice and rats. In fact, although a CR diet considerably extends lifespan in these organisms, even if it is implemented at the age at which skeletal development is complete, the maximal benefit of this low-calorie diet for longevity can be achieved only if CR is initiated during the rapid growth period.Citation29-Citation31 These findings suggest that laboratory mice and rats (1) could employ a CR-dependent longevity control system that, by monitoring some key, longevity-defining cellular processes, can establish a particular rate of cellular and organismal aging; and (2) could have at least two checkpoints, one in early adulthood and another in late adulthood, at which the proposed CR-dependent longevity control system senses the rate and/or efficiency of the critical cellular processes that define longevity. It is conceivable therefore that the proposed CR-dependent longevity control system in laboratory mice and rats can extend longevity even if the rate and efficiency of the critical, CR-modulated cellular processes are appropriate only at the late-adulthood checkpoint, but not as markedly as if they are suitable already at the checkpoint in early adulthood.
According to the hypothesis proposed here for a stepwise development of a longevity network configuration at a series of checkpoints, a pharmacological anti-aging intervention may modulate the key longevity-defining cellular processes that are monitored at a particular checkpoint by a master regulator of the longevity control system. Several anti-aging compounds are currently known for their ability to extend longevity across phyla and improve health by beneficially influencing age-related pathologies (for a comprehensive list of such compounds and mechanisms underlying their longevity-extending effects, see Table S1 in ref. Citation33).Citation2,Citation32-Citation34 Only one of these anti-aging compounds, a macrocyclic lactone rapamycin synthesized by soil bacteria to inhibit growth of fungal competitors within an ecosystem,Citation35 has been examined for its effects on lifespan and healthspan if implemented at different ages of an organism. If fed to genetically heterogeneous mice beginning at 270 d or 600 d of age, this specific inhibitor of the nutrient-sensory protein kinase mTORC1 (mammalian target of rapamycin complex 1) has been shown to be equally efficient in extending lifespan and beneficially influencing age-related pathologies.Citation32,Citation36-Citation39 Our recent studies provided evidence that (1) LCA, a bile acid, extends longevity of chronologically aging yeast if added to growth medium at the time of cell inoculation;Citation33 and (2) longevity in chronologically aging yeast is programmed by the level of metabolic capacity and organelle organization that they developed, in a diet-specific fashion, before entering a quiescent state and, thus, that chronological aging in yeast is likely to be the final step of a developmental program progressing through at least one checkpoint prior to entry into quiescence.Citation10 We therefore sought to investigate how LCA influences longevity and a compendium of longevity-defining cellular processes in chronologically aging yeast if added to growth medium at different periods of lifespan. We found that LCA can extend longevity of CR yeast only if added at either of two critical lifespan periods. One of them includes logarithmic and diauxic growth phases, whereas the other period exists in early stationary phase. Our analysis of how the addition of LCA to CR yeast at different periods of chronological lifespan influences several longevity-extending and longevity-shortening processes suggests a mechanism that may link the ability of LCA to increase the lifespan of CR yeast only if added at either of the two critical periods to its differential effects on a set of longevity-defining processes monitored in this study. In our model for this mechanism, LCA controls these longevity-defining processes at three checkpoints that exist in logarithmic/diauxic, post-diauxic and early stationary phases. Based on these findings, we propose a hypothesis on a stepwise progression of a biomolecular longevity network through a series of checkpoints, at each of which (1) some genetic, dietary and pharmacological anti-aging interventions modulate certain longevity-defining processes within modules comprising the longevity network; and (2) some checkpoint-specific master regulators monitor and govern the functional states of these critical network modules.
Results
LCA increases the chronological lifespan of yeast cultured under CR or non-CR conditions only if added at certain critical periods
We sought to examine if the addition of LCA to chronologically aging yeast at different periods of lifespan has an effect on the longevity-extending efficacy of this bile acid. Yeasts were cultured in YP medium under CR conditions on 0.2% glucose or under non-CR conditions on 2% glucose, and LCA was added to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. LCA was used at the final concentration of 50 µM, at which this natural anti-aging compound has been shown to exhibit the greatest beneficial effect on longevity of chronologically aging yeast under both CR and non-CR conditions.Citation33
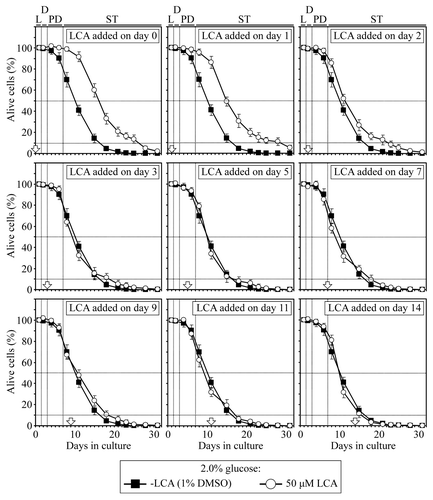
We found that in yeast cultured under CR conditions on 0.2% glucose, there are two critical periods when the addition of LCA to growth medium can increase both their mean and maximum chronological lifespans ( and ). One of these two critical periods, which we term period 1, includes L (logarithmic) and D (diauxic) growth phases. The other critical period, which is called period 3, exists in early ST (stationary) phase. In contrast, LCA did not cause a significant extension of the mean or maximum chronological lifespan of CR yeast if it was added at period 2 or 4 that exists in PD (post-diauxic) or late ST phase, respectively ( and ).
Figure 1. In yeast cultured under CR conditions on 0.2% glucose, there are two critical periods when the addition of LCA to growth medium can extend longevity. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Chronological lifespan analysis was performed as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 8–12). Abbreviations, D, diauxic; L, logarithmic; PD, post-diauxic; ST, stationary phase.
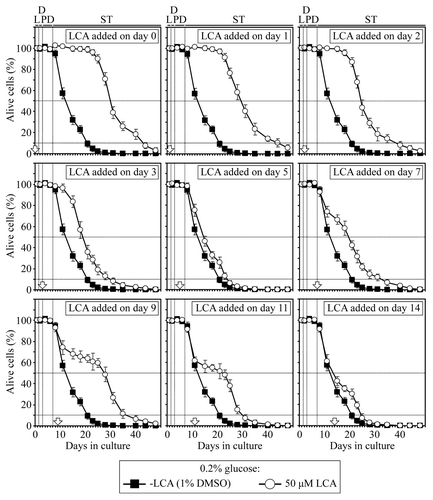
Figure 3. In yeast cultured under CR conditions on 0.2% glucose, there are two critical periods when the addition of LCA to growth medium can extend longevity. In yeast cultivated under non-CR conditions on 2% glucose, there is only one such a period. The mean (A) and maximum (B) chronological lifespans of different wild-type yeast cultures are shown. Wild-type yeast cells were cultured under CR or non-CR conditions and LCA was added as described in the legends for and , respectively. Chronological lifespan analysis was performed as described in “Materials and Methods.” Data are presented as mean ± SEM [n = 8–12 for (A); n = 6–7 for (B)]; *p < 0.05 (relative to the mean or maximum chronological lifespan of yeast not exposed to LCA).
![Figure 3. In yeast cultured under CR conditions on 0.2% glucose, there are two critical periods when the addition of LCA to growth medium can extend longevity. In yeast cultivated under non-CR conditions on 2% glucose, there is only one such a period. The mean (A) and maximum (B) chronological lifespans of different wild-type yeast cultures are shown. Wild-type yeast cells were cultured under CR or non-CR conditions and LCA was added as described in the legends for Figures 1 and 2, respectively. Chronological lifespan analysis was performed as described in “Materials and Methods.” Data are presented as mean ± SEM [n = 8–12 for (A); n = 6–7 for (B)]; *p < 0.05 (relative to the mean or maximum chronological lifespan of yeast not exposed to LCA).](/cms/asset/ac415b2f-61e5-4ea0-8fe6-d16e7868e326/kccy_a_10921754_f0002.gif)
We also found that in yeast cultivated under non-CR conditions on 2% glucose, there is only one critical period, during L and D phases, when the addition of LCA to growth medium can increase both their mean and maximum chronological lifespans ( and ). However, if LCA was added to non-CR yeast at any time-point after this critical period ended, it did not significantly alter their mean or maximum chronological lifespan ( and ). Thus, unlike a substantial beneficial effect of LCA on yeast longevity seen if it was added in early ST phase under CR conditions, this bile acid was unable to extend longevity of chronologically aging yeast under non-CR conditions if added in the same phase.
Figure 2. In yeast cultured under non-CR conditions on 2% glucose, there is only one critical period when the addition of LCA to growth medium can extend longevity. Wild-type yeast cells were cultured in YP medium initially containing 2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Chronological lifespan analysis was performed as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 6–7).
There are several ways through which LCA could differentially influence longevity if added to CR yeast at different periods of their lifespan
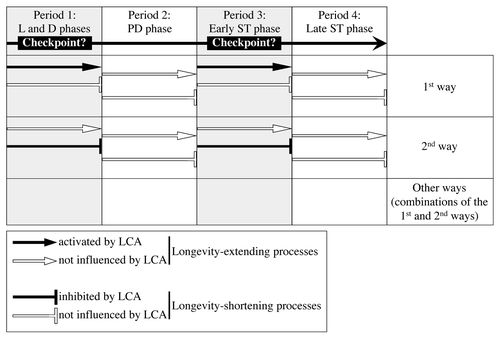
Aging of multicellular and unicellular eukaryotic organisms affects numerous longevity-defining processes within cells.Citation1-Citation4,Citation34,Citation40 It is conceivable, therefore, that the observed ability of LCA to extend longevity of chronologically aging yeast under CR conditions only if added at period 1 or 3 of their lifespan ( and ) could be due to its differential and, perhaps, enduring effects on certain longevity-extending and longevity-shortening processes controlled at different lifespan periods. One could envisage several ways through which LCA can differentially influence these longevity-defining processes following its addition at different periods of yeast chronological lifespan (). For example, LCA could activate a longevity-extending process (or several processes) controlled at certain checkpoints that may exist in L/D and early ST growth phases (periods 1 and 3, respectively), without influencing longevity-shortening processes at these envisioned lifespan checkpoints or having an effect on any longevity-defining processes during periods 2 and 4 in PD and late ST phases (; see the first way). Alternatively, LCA could inhibit a longevity-shortening process (or several processes) controlled at certain checkpoints that may exist in L/D and early ST phases (periods 1 and 3, respectively), without influencing longevity-extending processes at these envisioned lifespan checkpoints or having an effect on any longevity-defining processes during periods 2 and 4 in PD and late ST phases (; see the second way). Moreover, the observed ability of LCA to extend longevity of chronologically aging yeast under CR conditions only if added at periods 1 and 3 of their lifespan could be also due to various combinations of the first and the second ways outlined above (; see other ways). We therefore sought to examine how the addition of LCA at different periods of chronological lifespan in yeast cultured under CR conditions influences longevity-extending and longevity-shortening processes controlled during each of these periods.
Figure 4. There are several ways through which LCA could differentially influence some longevity-extending and longevity-shortening processes following its addition at different periods of yeast chronological lifespan. LCA could control these longevity-defining processes at certain checkpoints that may exist in L/D and early ST phases (periods 1 and 3, respectively). PD and late ST phases constitute periods 2 and 4, respectively. See text for details.
LCA makes yeast cells resistant to mitochondria-controlled apoptotic death, a longevity-shortening process, only if added at period 1, 2 or 3 of their chronological lifespan
A short-term exposure of yeast to hydrogen peroxide, acetic acid, hyperosmotic stress, α pheromone or several other exogenous agents causes apoptotic cell death that has been linked to mitochondrial fragmentation, mitochondrial outer membrane permeabilization and the release of several intermembrane space proteins from mitochondria.Citation41-Citation44 The exit of Aif1p (apoptosis inducing factor 1) and Nuc1p (endonuclease G) from yeast mitochondria and their subsequent import into the nucleus trigger such exogenously induced apoptosis by promoting DNA cleavage.Citation45,Citation46 Another intermembrane space protein that is released from yeast mitochondria during exogenously induced apoptosis is cytochrome c.Citation42,Citation47,Citation48 Akin to its essential role in triggering the apoptotic caspase cascade in mammalian cells,Citation49 cytochrome c in the cytosol of yeast cells activates the metacaspase Yca1p.Citation42,Citation50-Citation52 Importantly, chronologically aging yeast die in an Aif1p-, Nuc1p- and Yca1p-dependent fashion, exhibiting characteristic markers of apoptosis such as chromatin condensation, nuclear fragmentation, DNA cleavage, phosphatidylserine externalization, ROS (reactive oxygen species) production and caspase activation.Citation45,Citation46,Citation53-Citation56 Thus, the chronological aging of yeast is linked to an apoptosis-like, mitochondria-controlled programmed cell death.Citation57-Citation61 It should be emphasized that mutations eliminating pro-apoptotic proteins as well as such potent anti-aging interventions as a CR diet and LCA, (1) extend longevity of chronologically aging yeast; (2) delay age-related apoptotic death controlled by mitochondria and (3) reduce the susceptibility of yeast to cell death triggered by a short-term exposure to exogenous hydrogen peroxide and known to be caused by mitochondria-controlled apoptosis.Citation10,Citation33,Citation41,Citation43,Citation45,Citation46,Citation53-Citation62
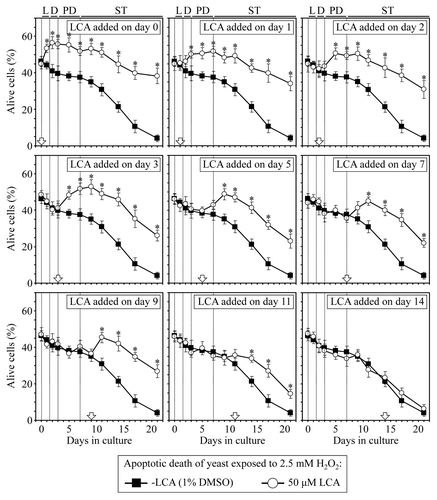
Taken together, these findings strongly support the notion that mitochondria-controlled apoptotic death plays an essential role in regulating longevity of chronologically aging yeast. This form of longevity-defining cell death can be triggered by a brief exposure of yeast to exogenous hydrogen peroxide.Citation10,Citation33,Citation41,Citation43,Citation62 We therefore examined how the addition of LCA at different periods of chronological lifespan influences a longevity-shortening process of mitochondria-controlled apoptotic cell death in yeast cultured under CR on 0.2% glucose. We monitored the susceptibility of yeast to cell death triggered by a short-term (for 2 h) exposure to exogenous hydrogen peroxide known to cause mitochondria-controlled apoptosis. We found that if added to growth medium on day 0, 1, 2, 3, 5, 7, 9 or 11, LCA reduces the susceptibility of CR yeast to apoptosis induced by a brief exposure to exogenous hydrogen peroxide following LCA addition; this effect of LCA persists through the rest of the lifespan (). In contrast, if added to growth medium on day 14, LCA does not alter the susceptibility of CR yeast to this form of apoptotic cell death for the remainder of the lifespan (). Thus, LCA makes yeast cells resistant to mitochondria-controlled apoptotic death, a longevity-shortening process, only if added at period 1, 2 or 3 of chronological lifespan. This effect of LCA persists long after each of these lifespan periods ended.
Figure 5. LCA reduces the susceptibility of yeast cells to mitochondria-controlled apoptotic death, a longevity-shortening process, only if added at period 1, 2 or 3 of chronological lifespan and for the rest of the lifespan. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Cell viability assay for monitoring the susceptibility of yeast to an apoptotic mode of cell death induced by a 2 h exposure to exogenous hydrogen peroxide was performed as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 3–5); *p < 0.05 (relative to the % of alive cells in yeast cultures not exposed to LCA).
LCA differentially influences the susceptibility of chronologically aging yeast to palmitoleic acid-induced necrotic cell death, a longevity-shortening process, if added at different periods of their lifespan
In our recently proposed model for a mechanism linking yeast longevity and lipid dynamics in the endoplasmic reticulum, lipid bodies and peroxisomes, a remodeling of lipid metabolism in chronologically aging non-CR yeast shortens their lifespan by causing premature death in part due to necrotic cell death triggered by the accumulation of free fatty acids.Citation10,Citation33,Citation63-Citation65 Importantly, both CR and LCA not only extend longevity of chronologically aging yeast but also reduce their susceptibility to a form of necrotic cell death triggered by a short-term exposure to exogenous palmitoleic fatty acid.Citation10,Citation33,Citation63-Citation65 These findings imply that palmitoleic acid-induced necrotic cell death plays an essential role in regulating longevity of chronologically aging yeast. We therefore assessed how the addition of LCA at different periods of chronological lifespan influences a longevity-shortening process of necrotic cell death in yeast cultured under CR on 0.2% glucose. We examined the susceptibility of yeast to cell death triggered by a short-term (for 2 h) exposure to exogenous palmitoleic acid. We found that if added to growth medium on day 0, 1 or 2, LCA reduces the susceptibility of CR yeast to necrosis induced by a brief exposure to palmitoleic acid following LCA addition; this effect of LCA persists through the remainder of the lifespan (). In contrast, LCA either does not influence (if added on day 3, 9, 11 or 14) or increases for the rest of the lifespan (if added on day 5 or 7) the susceptibility of CR yeast to this form of necrotic cell death ().
Figure 6. LCA differentially influences the susceptibility of chronologically aging yeast to palmitoleic acid-induced necrotic cell death, a longevity-shortening process, if added at different periods of lifespan. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Cell viability assay for monitoring the susceptibility of yeast to a necrotic mode of cell death induced by a 2 h exposure to exogenous palmitoleic acid was performed as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 4); *p < 0.05 (relative to the % of alive cells in yeast cultures not exposed to LCA).
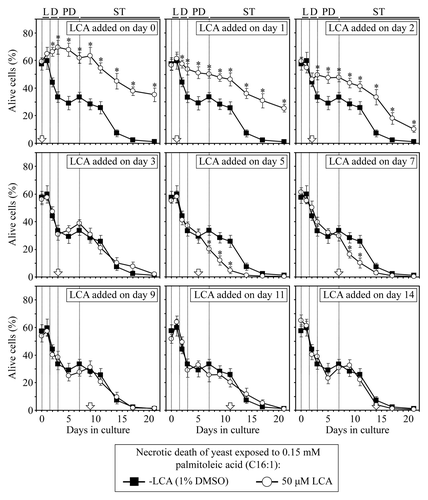
Thus, LCA makes yeast cells resistant to palmitoleic acid-induced necrotic death, a longevity-shortening process, only if added at period 1 that includes L and D growth phases; this effect of LCA persists long after the end of period 1. It is conceivable that the observed inability of LCA to extend yeast longevity if added at period 2 of chronological lifespan ( and ) could be due to the observed stimulating lifelong effect of LCA on a palmitoleic acid-induced form of necrotic cell death if added at this period in PD phase (). Furthermore, despite LCA extends longevity of chronologically aging yeast if added at period 3, it does not affect their susceptibility to necrotic cell death if added at this period in early ST phase (). Moreover, the observed lack of an effect of LCA on yeast longevity if added at period 4 of chronological lifespan (, and ) coincides with its inability to alter cell susceptibility to necrotic cell death if added at this period in late ST phase ().
LCA differentially influences a longevity-extending process of the maintenance of nuclear and mitochondrial genomes if added at different periods of yeast’s chronological lifespan
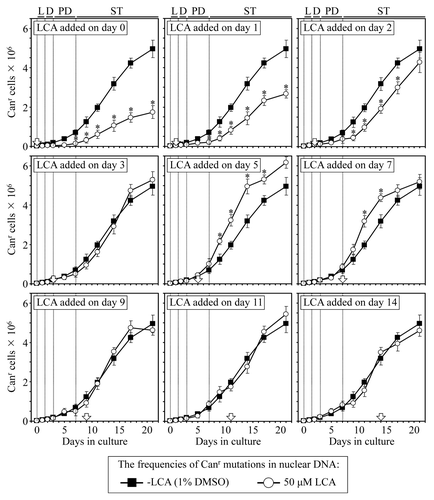
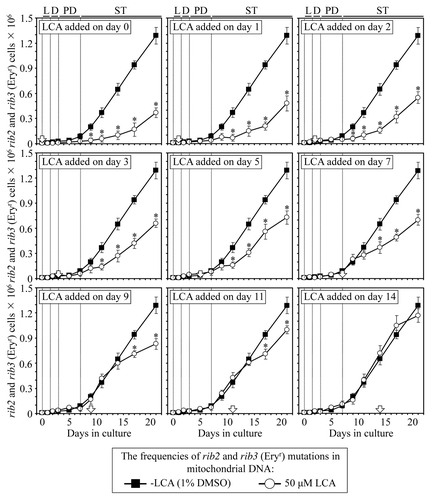
A body of evidence supports the view that the maintenance of nDNA (nuclear DNA) and mtDNA (mitochondrial DNA) integrity is an essential longevity-extending process in evolutionarily distant organisms, including yeast.Citation10,Citation33,Citation40,Citation66-Citation68 We therefore examined how the addition of LCA to yeast cultured under CR on 0.2% glucose at different periods of chronological lifespan influences (1) the frequency of spontaneous point mutations in the CAN1 gene of nDNA, (2) the frequencies of spontaneous single-gene (mit- and syn-) and deletion (rho- and rhoo) mutations in mtDNA, all causing a deficiency in mitochondrial respiration and impairing growth on glycerol and (3) the frequencies of spontaneous point mutations in the rib2 and rib3 loci of mtDNA.
We found that if added to growth medium on day 0, 1 or 2, LCA reduces the frequency of spontaneous point mutations in the CAN1 gene of nDNA for the rest of the lifespan (). In contrast, LCA either does not influence (if added on day 3, 9, 11 or 14) or increases for the remainder of the lifespan (if added on day 5 or 7) the frequency of these spontaneous point mutations in nDNA (). Thus, LCA stimulates the maintenance of nDNA integrity, an essential longevity-extending process, only if added at period 1, which includes L and D growth phases; this effect of LCA persists long after the end of period 1. Our findings also suggest that the observed lifelong inhibitory effect of LCA on the maintenance of nDNA integrity if it is added at period 2 of yeast chronological lifespan () could in part be responsible for the inability of LCA to extend yeast longevity if added at this period in PD phase (, and ). Furthermore, despite that LCA extends longevity of chronologically aging yeast if added at period 3, it does not influence the maintenance of nDNA integrity if added at this period during early ST phase (). Moreover, the observed lack of an effect of LCA on yeast longevity if added at period 4 of chronological lifespan (, and ) coincides with the inability of this compound to alter the efficacy of the maintenance of nDNA integrity if added at this period in late ST phase ().
Figure 7. LCA differentially influences the maintenance of nDNA integrity, an essential longevity-extending process, if added at different periods of yeast lifespan. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). The frequency of spontaneous point mutations in the CAN1 gene of nDNA was measured as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 3); *p < 0.05 (relative to the frequency of spontaneous point mutations in the CAN1 gene of nDNA in yeast cultures not exposed to LCA).
We also found that if added to growth medium on day 0, 1, 2, 3, 5, 7, 9 or 11, LCA reduces for the rest of the lifespan (1) the frequencies of spontaneous single-gene (mit- and syn-) and deletion (rho- and rhoo) mutations in mtDNA, all causing a deficiency in mitochondrial respiration and impairing growth on glycerol (); and (2) the frequencies of spontaneous point mutations in the rib2 and rib3 loci of mtDNA (). In contrast, if added to growth medium on day 14, LCA does not affect the frequencies of these spontaneous mutations in mtDNA ( and ). Thus, LCA stimulates the maintenance of mtDNA integrity, an essential longevity-extending process, only if added at period 1, 2 or 3 that exists in L/D, PD or early ST phase (respectively); this effect of LCA persists long after the end of each of these lifespan periods. Moreover, the observed lack of an effect of LCA on yeast longevity if added at period 4 of chronological lifespan (, and ) coincides with the inability of this compound to alter the efficacy of the maintenance of mtDNA integrity if added at this period in late ST phase ( and ).
Figure 8. LCA differentially influences the maintenance of mtDNA integrity, an essential longevity-extending process, if added at different periods of yeast lifespan. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). The frequencies of spontaneous single-gene (mit- and syn-) and deletion (rho- and rhoo) mutations in mtDNA, all causing a deficiency in mitochondrial respiration and impairing growth on glycerol, were measured as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 5–6); *p < 0.05 (relative to the frequencies of spontaneous mit-, syn-, rho- and rhoo mutations in mtDNA in yeast cultures not exposed to LCA).
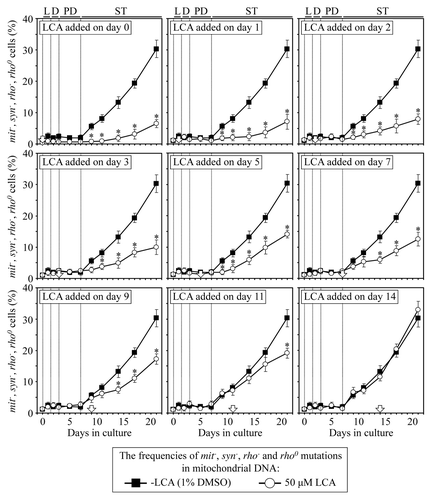
Figure 9. LCA differentially influences the maintenance of mtDNA integrity, an essential longevity-extending process, if added at different periods of yeast lifespan. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). The frequencies of spontaneous point mutations in the rib2 and rib3 loci of mtDNA were measured as described in “Materials and Methods.” Data are presented as mean ± SEM (n = 3–4); *p < 0.05 (relative to the frequencies of spontaneous rib2 and rib3 mutations in mtDNA in yeast cultures not exposed to LCA).
LCA differentially influences a longevity-extending process of the development of resistance to chronic stresses if added at different periods of yeast’s chronological lifespan
It is well established that the development of resistance to chronic (long-term) oxidative, thermal and osmotic stresses is an essential longevity-extending process in evolutionarily distant organisms, including yeast.Citation2,Citation4,Citation10,Citation33,Citation34,Citation40,Citation69-Citation73 We therefore examined how the addition of LCA to yeast cultured under CR on 0.2% glucose at different periods of chronological lifespan influences their resistance to each of these chronic stresses. We found that if added to growth medium on day 0, 1, 2 or 3, LCA increases for the remainder of the lifespan the resistance of yeast to chronic oxidative and thermal stresses, but does not alter cell susceptibility to chronic osmotic stress (, and ; Figs. S1–4). Moreover, LCA increases the resistance of yeast to all three chronic stresses if added to growth medium on day 7, 9 or 11; this effect of LCA persists long after its addition (–; Figs. S1–4). In contrast, LCA does not alter cell susceptibility to any of these chronic stresses if added on day 5 or 14 (–; Figs. S1–4).
Figure 10. Effect of LCA added at different periods of chronological lifespan on the ability of yeast to resist chronic oxidative stress. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Spot assays for monitoring oxidative stress resistance were performed as described in “Materials and Methods.” Serial 10-fold dilutions of cells were spotted on plates with solid YP medium containing 2% glucose as carbon source and 5 mM hydrogen peroxide. All pictures were taken after a 3-d incubation at 30°C.
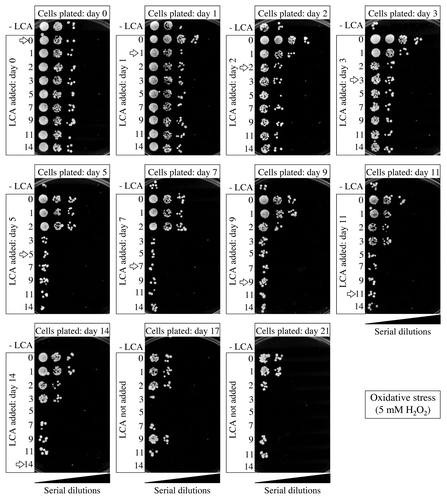
Figure 11. Effect of LCA added at different periods of chronological lifespan on the ability of yeast to resist chronic thermal stress. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Spot assays for monitoring thermal stress resistance were performed as described in “Materials and Methods.” Serial 10-fold dilutions of cells were spotted on plates with solid YP medium containing 2% glucose as carbon source. Plates were initially incubated at 55°C for 30 min, and were then transferred to 30°C. All pictures were taken after a 3-d incubation at 30°C.

Figure 12. Effect of LCA added at different periods of chronological lifespan on the ability of yeast to resist chronic osmotic stress. Wild-type yeast cells were cultured in YP medium initially containing 0.2% glucose, and LCA was added at the final concentration of 50 µM to a cell culture immediately following cell inoculation into the medium (on day 0) or on day 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in this medium. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with compound vehicle) was 1% (v/v). Spot assays for monitoring osmotic stress resistance were performed as described in “Materials and Methods.” Serial 10-fold dilutions of cells were spotted on plates with solid YP medium containing 2% glucose as carbon source and 1 M sorbitol. All pictures were taken after a 3-d incubation at 30°C.

Thus, in chronologically aging yeast, LCA stimulates a longevity-extending process of the development of lifelong resistance to chronic oxidative and thermal stresses if added at period 1 that exists in L and D phases. If added at period 3 (which includes early ST phase), LCA not only stimulates the development of resistance to chronic oxidative and thermal stresses for the rest of the lifespan, but also enhances a longevity-extending process of developing enduring resistance to chronic osmotic stress. If added at any of these two periods, LCA can extend longevity (, and ). Noteworthy, the observed lack of an effect of LCA on yeast longevity if added at period 2 or period 4 of chronological lifespan (, and ) coincides with the inability of this compound to alter cell susceptibility to any of these stresses at these two periods in PD and late ST phases, respectively.
Discussion
This study provides evidence that LCA, a bile acid, can extend longevity of CR yeast only if added at period 1 (which includes L and D growth phases) or period 3 (which exists in early ST phase) of chronological lifespan (). In contrast, LCA is unable to extend the mean or maximum chronological lifespan of yeast cultured under CR conditions if added at period 2 or 4 in PD or late ST phase, respectively (). We also found that longevity of yeast cultivated under non-CR conditions can be extended by LCA only if it is added at period 1, but not following its addition at period 2, 3 or 4.
Figure 13. A mechanism that may link the ability of LCA to extend longevity of CR yeast only if added at period 1 or 3 of chronological lifespan to the differential effects of this compound on certain longevity-extending and longevity-shortening processes controlled at different lifespan periods. LCA may control these longevity-defining processes at the checkpoints A, B and C that exist in L/D (period 1), PD (period 2) and early ST (period 3) phases, respectively. See text for details.
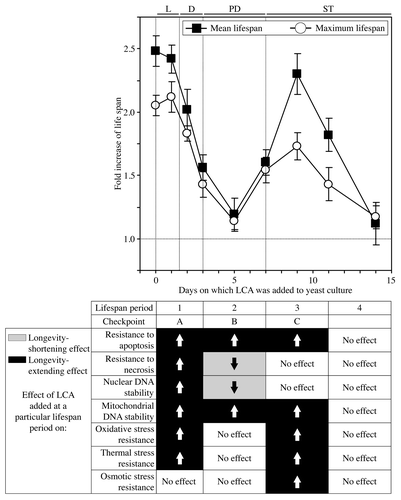
Our analysis of how the addition of LCA to CR yeast at different periods of chronological lifespan influences several longevity-extending and longevity-shortening processes suggests a mechanism that may link the ability of LCA to increase the lifespan of CR yeast only if added at period 1 or 3 to its differential effects on a monitored [AUTHOR’S NOTE: Missing word here?] in this study set of longevity-defining processes controlled at different lifespan periods (). In this mechanism, LCA added to CR yeast at period 1 increases chronological lifespan because (1) its addition at this period has a beneficial effect on six longevity-defining processes; (2) the longevity-extending effect of added [AUTHOR’S NOTE: Missing word here?] at this period LCA on each of these processes persists through the entire lifespan, long after this critical lifespan period ended and (3) the addition of LCA at this period does not cause a lifespan-shortening effect on any of the longevity-defining processes monitored in this study (). Furthermore, although the addition of LCA at period 2 imposes a lifelong beneficial effect on two longevity-defining cellular process (i.e., it enhances cell resistance to mitochondria-controlled apoptotic death and stimulates mitochondrial genome maintenance), the observed inability of the compound to extend longevity if added at this period of lifespan could be due to the two enduring longevity-shortening effects that LCA exhibits following its addition at period 2 (). These detrimental to longevity effects of LCA added at period 2 include a lifelong reduction of cell resistance to palmitoleic acid-induced necrotic death and a long-term decline of the efficacy with which the integrity of nuclear genome is maintained (). In the mechanism that we propose here, LCA added to CR yeast at period 3 increases chronological lifespan because (1) its addition at this period has a beneficial effect on five longevity-defining processes for the rest of the lifespan; and (2) the addition of LCA at this period does not cause a lifespan-shortening effect on any of the longevity-defining processes that we monitored (). Moreover, the observed lack of an effect of LCA on a monitored set of longevity-defining processes following its addition at period 4 may satisfactorily explain the inability of LCA to extend longevity if added at this lifespan period ().
Our model for a mechanism that may link the ability of LCA to increase the lifespan of CR yeast only if added at period 1 or 3 to its differential effects on several longevity-defining processes also foresees that LCA controls these processes at the checkpoints A, B and C that exist in L/D (period 1), PD (period 2) and early ST (period 3) phases, respectively (). It is conceivable that at each of these checkpoints LCA modulates the key set of longevity-defining cellular processes (modules) that comprise a biomolecular longevity network and are monitored by certain master regulators of the longevity control system. Based on the information gathered and processed by the master regulators, they modulate certain longevity-defining processes within monitored modules of the longevity network in order to limit the age-related accumulation of molecular and cellular damage. The resulting changes in the dynamics of individual modules constituting the network and in its general configuration are critically important for establishing the rate of cellular aging and, thus, define longevity.
Recent studies support the validity of the proposed here hypothesis on a stepwise progression of a biomolecular longevity network through a series of checkpoints, at each of which (1) some genetic, dietary and pharmacological anti-aging interventions modulate certain longevity-defining processes within modules comprising the longevity network; and (2) some checkpoint-specific master regulators monitor and govern the functional states of these critical network modules.
Studies in chronologically aging yeast revealed that, by promoting coupled respiration in mitochondria, elevating the mitochondrial membrane potential and increasing mitochondrial ROS production during L phase, a CR diet and some pharmacological interventions extend longevity by causing changes in several longevity-defining processes during the subsequent D, PD and ST phases. These changes include (1) increased intracellular levels of trehalose and glycogen, the two major glucose stores of yeast; (2) a remodeling of lipid metabolism in the endoplasmic reticulum, lipid bodies and peroxisomes; (3) reduced cell susceptibility to age-related forms of mitochondria-controlled apoptotic and lipid-induced necrotic death; (4) an attenuation of mitochondrial fragmentation; (5) a reduction in the mitochondrial membrane potential and mitochondrial ROS production; (6) elevated stability of nuclear and mitochondrial genomes and (7) enhanced resistance to chronic oxidative and thermal stresses.Citation10,Citation33,Citation74-Citation77 It seems that in chronologically aging yeast, TORC1 operates as one of the predicted by our hypothesis, checkpoint-specific master regulators that at a checkpoint in L phase can monitor and govern a functional state of mitochondria.Citation74-Citation77
Furthermore, recent studies in chronologically aging yeast suggest the existence of two checkpoints at which the intracellular level of trehalose defines longevity by modulating cellular proteostasis throughout lifespan.Citation10,Citation78 At one of these lifespan checkpoints in PD phase, trehalose operates as an anti-aging compound that (1) stabilizes the native state of proteins and thereby reduces the formation of their aberrantly folded species; (2) reduces the formation of insoluble protein aggregates by shielding the contiguous exposed hydrophobic side chains of amino acids that are abundant in misfolded, partially folded and unfolded protein species and promote their aggregation and (3) protects cellular proteins from oxidative carbonylation by interacting with their carbonylation-prone misfolded and unfolded species.Citation78 At another lifespan checkpoint in ST phase, trehalose functions as a pro-aging compound that shields the contiguous exposed hydrophobic side chains of amino acids abundant in misfolded, partially folded and unfolded protein species. By competing with molecular chaperones for binding with these patches of hydrophobic amino acid residues, trehalose interferes with the essential longevity-extending process of chaperone-assisted refolding of aberrantly folded protein species.Citation78
Moreover, as it has been mentioned in the “Introduction” section, studies in the nematode C. elegans provided evidence that UBL-5/DVE-1, DAF-16 and PHA-4 operate as the checkpoint-specific master regulators of longevity by governing progression through the three consecutive checkpoints operating during the L3/L4 larval stage of development, early adulthood and late adulthood, respectively.Citation21-Citation28
Taken together, these findings support the view that aging in organisms across phyla is the final step of a developmental program whose progression through several lifespan checkpoints in a genotype-specific fashion is modulated by environmental cues (such as caloric and dietary intake, environmental stresses, endocrine factors, etc.) and is both monitored and governed by an evolutionarily conserved set of checkpoint-specific master regulators. Other data supporting this view have been comprehensively discussed elsewhere.Citation10,Citation35,Citation54,Citation78-Citation85
The major challenge now is to get a greater insight into mechanisms that in chronologically aging yeast underly (1) a stepwise progression of the biomolecular longevity network through a series of checkpoints; (2) a modulation of various longevity-defining processes comprising the longevity network by genetic, dietary and pharmacological anti-aging interventions administered at different checkpoints and (3) a monitoring of these longevity-defining processes at each checkpoint by specific master regulators. To address this challenge, several important questions need to be answered. How will genetic, dietary and pharmacological anti-aging interventions known to directly target specific longevity-extending or longevity-shortening processes alter the age-related dynamics of changes in the proteomes, lipidomes and metabolomes of chronologically aging yeast? How will these interventions affect the chronology of other longevity-defining processes that comprise the longevity network but are known not to be directly modulated by these interventions? How will genetic and pharmacological anti-aging interventions that specifically modulate the functional states of several currently known master regulators of yeast longevity influence a timeline of changes in the proteomes, lipidomes and metabolomes of chronologically aging yeast and what will be their effects on the age-related dynamics of various longevity-defining processes comprising the longevity network? We shall have to answer these important questions if we want to understand the complexity of the biomolecular network whose progression through a series of checkpoints is modulated by various environmental cues and is governed by checkpoint-specific master regulators.
Materials and Methods
Strains and media
The wild-type strain Saccharomyces cerevisiae BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) (Thermo Scientific/Open Biosystems; #YSC1054) was grown in YP medium (1% yeast extract, 2% peptone) (Fisher Scientific; #BP1422–2 and #BP1420–2, respectively) containing 0.2% or 2% glucose (Fisher Scientific; #D16–10) as carbon source. Cells were cultured at 30°C with rotational shaking at 200 rpm in Erlenmeyer flasks at a “flask volume/medium volume” ratio of 5:1.
Chronological lifespan assay
A sample of cells was taken from a culture at a certain time-point. A fraction of the sample was diluted in order to determine the total number of cells using a hemacytometer (Fisher Scientific; #0267110). Another fraction of the cell sample was diluted, and serial dilutions of cells were plated in duplicate onto YP plates containing 2% glucose as carbon source. After 2 d of incubation at 30°C, the number of colony-forming units (CFU) per plate was counted. The number of CFU was defined as the number of viable cells in a sample. For each culture, the percentage of viable cells was calculated as follows: (number of viable cells per ml/total number of cells per ml) × 100. The percentage of viable cells in mid-logarithmic phase was set at 100%. The lifespan curves were validated using a LIVE/DEAD yeast viability kit (Life Technologies/Invitrogen; #L-7009) following the manufacturer's instructions.
Pharmacological manipulation of chronological lifespan
Chronological lifespan analysis was performed as described above in this section. The lithocholic (LCA) (#L6250) bile acid was from Sigma. The stock solution of LCA in DMSO was made on the day of adding this compound to cell cultures. LCA was added to growth medium at the final concentration of 50 µM immediately following cell inoculation into the medium or on days 1, 2, 3, 5, 7, 9, 11 or 14 of cell culturing in the medium, as indicated. The final concentration of DMSO in yeast cultures supplemented with LCA (and in the corresponding control cultures supplemented with drug vehicle) was 1% (v/v).
Cell viability assay for monitoring the susceptibility of yeast to an apoptotic mode of cell death induced by hydrogen peroxide
A sample of cells was taken from a culture at a certain time-point. A fraction of the sample was diluted in order to determine the total number of cells using a hemacytometer. Two × 107 cells were harvested by centrifugation for 1 min at 21,000 × g at room temperature and resuspended in 2 ml of YP medium containing 0.2% glucose as carbon source. Each cell suspension was divided into two equal aliquots. One aliquot was supplemented with hydrogen peroxide (Fisher Scientific; #H325–500) to the final concentration of 2.5 mM, whereas other aliquot remained untreated. Both aliquots were then incubated for 2 h at 30°C on a Labquake rotator (Thermolyne/Barnstead International; #400110) set for 360° rotation. Serial dilutions of cells were plated in duplicate onto plates containing YP medium with 2% glucose as carbon source. After 2 d of incubation at 30°C, the number of colony-forming units (CFU) per plate was counted. The number of CFU was defined as the number of viable cells in a sample. For each aliquot of cells exposed to hydrogen peroxide, the percent of viable cells was calculated as follows: (number of viable cells per ml in the aliquot exposed to hydrogen peroxide/number of viable cells per ml in the control aliquot that was not exposed to hydrogen peroxide) × 100.
Cell viability assay for monitoring the susceptibility of yeast to a necrotic mode of cell death induced by palmitoleic acid
A sample of cells was taken from a culture at a certain time-point. A fraction of the sample was diluted in order to determine the total number of cells using a hemacytometer. Two × 107 cells were harvested by centrifugation for 1 min at 21,000 × g at room temperature and resuspended in 2 ml of YP medium containing 0.2% glucose as carbon source. Each cell suspension was divided into two equal aliquots. One aliquot was supplemented with palmitoleic acid (Sigma; #P9417) from a 50 mM stock solution (in 10% chloroform, 45% hexane and 45% ethanol) (Sigma; #650498, #248878 and #34852, respectively); the final concentration of palmitoleic acid was 0.15 mM (in 0.03% chloroform, 0.135% hexane and 0.135% ethanol). The other aliquot was supplemented with chloroform, hexane and ethanol added to the final concentrations of 0.03%, 0.135% and 0.135%, respectively. Both aliquots were then incubated for 2 h at 30°C on a Labquake rotator set for 360° rotation. Serial dilutions of cells were plated in duplicate onto plates containing YP medium with 2% glucose as carbon source. After 2 d of incubation at 30°C, the number of colony-forming units (CFU) per plate was counted. The number of CFU was defined as the number of viable cells in a sample. For each aliquot of cells exposed to palmitoleic acid, the percent of viable cells was calculated as follows: (number of viable cells per ml in the aliquot exposed to palmitoleic acid/number of viable cells per ml in the control aliquot that was not exposed to palmitoleic acid) × 100.
Measurement of the frequency of nuclear mutations
The frequency of spontaneous point mutations in the CAN1 gene of nuclear DNA was evaluated by measuring the frequency of mutations that caused resistance to the antibiotic canavanine.Citation10 A sample of cells was removed from each culture at various time-points. Cells were plated in triplicate onto YNB [0.67% yeast nitrogen base without amino acids (Fisher Scientific; #DF0919153)] plates containing 2% glucose and supplemented with L-canavanine (50 mg/L), histidine, leucine, lysine and uracil (Sigma; #C1625, #H8125, #L8912, #L5751 and #U0750, respectively). In addition, serial dilutions of each sample were plated in triplicate onto YP plates containing 2% glucose for measuring the number of viable cells. The number of CFU was counted after 4 d of incubation at 30°C. For each culture, the frequency of mutations that caused resistance to canavanine was calculated as follows: number of CFU per ml on YNB plates containing 2% glucose, L-canavanine (50 mg/L), histidine, leucine, lysine and uracil/number of CFU per ml on YP plates containing 2% glucose.
Measurement of the frequency of mitochondrial mutations affecting mitochondrial components
The frequency of spontaneous single-gene (mit- and syn-) and deletion (rho- and rhoo) mutations in mtDNA affecting essential mitochondrial components was evaluated by measuring the fraction of respiratory-competent (rho+) yeast cells remaining in their aging population. The rho+ cells maintained intact their mtDNA and their nuclear genes encoding essential mitochondrial components. Therefore, rho+ cells were able to grow on glycerol, a non-fermentable carbon source. In contrast, mutant cells deficient in mitochondrial respiration were unable to grow on glycerol. These mutant cells carried mutations in mtDNA (including single-gene mit- and syn- mutations or large deletions rho-) or completely lacked this DNA (rhoo mutants).Citation10 Serial dilutions of cell samples removed from different phases of growth were plated in triplicate onto YP plates containing either 2% glucose or 3% glycerol (Fisher Scientific; #BP229–4) as carbon source. Plates were incubated at 30°C. The number of CFU on YP plates containing 2% glucose was counted after 2 d of incubation, whereas the number of CFU on YP plates containing 3% glycerol was counted after 6 d of incubation. For each culture, the percentage of respiratory-deficient (mit-, syn-, rho-, rhoo and pet-) cells was calculated as follows: 100 - [(number of CFU per ml on YP plates containing 3% glycerol/number of CFU per ml on YP plates containing 2% glucose) × 100].
The frequency of spontaneous point mutations in the rib2 and rib3 loci of mtDNA was evaluated by measuring the frequency of mtDNA mutations that caused resistance to the antibiotic erythromycin.Citation10 These mutations impair only mtDNA.Citation10 A sample of cells was removed from each culture at various time-points. Cells were plated in triplicate onto YP plates containing 3% glycerol and erythromycin (1 mg/ml) (Acros Organics; #227330050). In addition, serial dilutions of each sample were plated in triplicate onto YP plates containing 3% glycerol as carbon source for measuring the number of respiratory-competent (rho+) cells. The number of CFU was counted after 6 d of incubation at 30°C. For each culture, the frequency of mutations that caused resistance to erythromycin was calculated as follows: number of CFU per ml on YP plates containing 3% glycerol and erythromycin/number of CFU per ml on YP plates containing 3% glycerol.
Plating assays for the analysis of resistance to various stresses
For the analysis of hydrogen peroxide (oxidative stress) resistance, serial dilutions (1:100 to 1:105) of cells removed from each culture at various time-points were spotted onto two sets of plates. One set of plates contained YP medium with 2% glucose alone, whereas the other set contained YP medium with 2% glucose supplemented with 5 mM hydrogen peroxide. Pictures were taken after a 3-d incubation at 30°C.
For the analysis of thermal stress resistance, serial dilutions (1:100 to 1:105) of wild-type and mutant cells removed from each culture at various time-points were spotted onto two sets of plates containing YP medium with 2% glucose. One set of plates was incubated at 30°C. The other set of plates was initially incubated at 55°C for 30 min, and was then transferred to 30°C. Pictures were taken after a 3-d incubation at 30°C.
For the analysis of osmotic stress resistance, serial dilutions (1:100 to 1:105) of wild-type and mutant cells removed from each culture at various time-points were spotted onto two sets of plates. One set of plates contained YP medium with 2% glucose alone, whereas the other set contained YP medium with 2% glucose supplemented with 1 M sorbitol (Sigma; S6021). Pictures were taken after a 3-d incubation at 30°C.
Statistical analysis
Statistical analysis was performed using Microsoft Excel’s (2010) Analysis ToolPack-VBA. All data are presented as mean ± SEM. The p values were calculated using an unpaired two-tailed t-test.
| Abbreviations: | ||
| Aif1p | = | apoptosis-inducing factor 1 |
| CR | = | caloric restriction |
| D | = | diauxic growth phase |
| DAF-16 | = | dauer formation protein 16 |
| DVE-1 | = | defective proventriculus protein 1 |
| IGF-1 | = | insulin-like growth factor 1 |
| L | = | logarithmic growth phase |
| LCA | = | lithocholic acid |
| mtDNA | = | mitochondrial DNA |
| mTORC1 | = | mammalian target of rapamycin complex 1 |
| nDNA | = | nuclear DNA |
| Nuc1p | = | endonuclease G |
| PD | = | post-diauxic growth phase |
| PHA-4 | = | pharynx development protein 4 |
| ROS | = | reactive oxygen species |
| ST | = | stationary growth phase |
| UBL-5 | = | ubiquitin-like protein 5 |
| UPRmt | = | mitochondria-specific unfolded protein response |
Additional material
Download Zip (649.6 KB)Acknowledgments
We acknowledge the Centre for Structural and Functional Genomics at Concordia University for outstanding services. This study was supported by grants from the NSERC of Canada and Concordia University Chair Fund. M.T.B. was supported by a Doctoral Research Fellowship Award from the FQRNT. P.K. was supported by Doctoral Research Fellowship Awards from the Fonds de recherché en santé du Quebec and from the Fonds québécois de la recherche sur la nature et les technologies (FQRNT). A.B. and V.R.R. were supported by Frederick Banting and Charles Best Doctoral Scholarship Awards from the Canadian Institutes of Health Research. V.I.T. is a Concordia University Research Chair in Genomics, Cell Biology and Aging.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Greer EL, Brunet A. Signaling networks in aging. J Cell Sci 2008; 121:407 - 12; http://dx.doi.org/10.1242/jcs.021519; PMID: 18256383
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science 2010; 328:321 - 6; http://dx.doi.org/10.1126/science.1172539; PMID: 20395504
- Kenyon CJ. The genetics of ageing. Nature 2010; 464:504 - 12; http://dx.doi.org/10.1038/nature08980; PMID: 20336132
- Longo VD, Shadel GS, Kaeberlein M, Kennedy B. Replicative and chronological aging in Saccharomyces cerevisiae.. Cell Metab 2012; 16:18 - 31; http://dx.doi.org/10.1016/j.cmet.2012.06.002; PMID: 22768836
- Kowald A, Kirkwood TB. Towards a network theory of ageing: a model combining the free radical theory and the protein error theory. J Theor Biol 1994; 168:75 - 94; http://dx.doi.org/10.1006/jtbi.1994.1089; PMID: 8022192
- Kowald A, Kirkwood TB. A network theory of ageing: the interactions of defective mitochondria, aberrant proteins, free radicals and scavengers in the ageing process. Mutat Res 1996; 316:209 - 36; http://dx.doi.org/10.1016/S0921-8734(96)90005-3; PMID: 8649456
- Kirkwood TB, Kowald A. Network theory of aging. Exp Gerontol 1997; 32:395 - 9; http://dx.doi.org/10.1016/S0531-5565(96)00171-4; PMID: 9315444
- Kirkwood TB, Boys RJ, Gillespie CS, Proctor CJ, Shanley DP, Wilkinson DJ. Towards an e-biology of ageing: integrating theory and data. Nat Rev Mol Cell Biol 2003; 4:243 - 9; http://dx.doi.org/10.1038/nrm1051; PMID: 12612643
- Murphy MP, Partridge L. Toward a control theory analysis of aging. Annu Rev Biochem 2008; 77:777 - 98; http://dx.doi.org/10.1146/annurev.biochem.77.070606.101605; PMID: 18318658
- Goldberg AA, Bourque SD, Kyryakov P, Gregg C, Boukh-Viner T, Beach A, et al. Effect of calorie restriction on the metabolic history of chronologically aging yeast. Exp Gerontol 2009; 44:555 - 71; http://dx.doi.org/10.1016/j.exger.2009.06.001; PMID: 19539741
- Witten M. A return to time, cells, systems and aging: II. Relational and reliability theoretic approaches to the study of senescence in living systems. Mech Ageing Dev 1984; 27:323 - 40; http://dx.doi.org/10.1016/0047-6374(84)90056-3; PMID: 6513610
- Kriete A, Sokhansanj BA, Coppock DL, West GB. Systems approaches to the networks of aging. Ageing Res Rev 2006; 5:434 - 48; http://dx.doi.org/10.1016/j.arr.2006.06.002; PMID: 16904954
- Budovsky A, Abramovich A, Cohen R, Chalifa-Caspi V, Fraifeld V. Longevity network: construction and implications. Mech Ageing Dev 2007; 128:117 - 24; http://dx.doi.org/10.1016/j.mad.2006.11.018; PMID: 17116322
- Curtis C, Landis GN, Folk D, Wehr NB, Hoe N, Waskar M, et al. Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol 2007; 8:R262; http://dx.doi.org/10.1186/gb-2007-8-12-r262; PMID: 18067683
- Xue H, Xian B, Dong D, Xia K, Zhu S, Zhang Z, et al. A modular network model of aging. Mol Syst Biol 2007; 3:147; http://dx.doi.org/10.1038/msb4100189; PMID: 18059442
- Managbanag JR, Witten TM, Bonchev D, Fox LA, Tsuchiya M, Kennedy BK, et al. Shortest-path network analysis is a useful approach toward identifying genetic determinants of longevity. PLoS ONE 2008; 3:e3802; http://dx.doi.org/10.1371/journal.pone.0003802; PMID: 19030232
- Barea F, Bonatto D. Aging defined by a chronologic-replicative protein network in Saccharomyces fcerevisiae: an interactome analysis. Mech Ageing Dev 2009; 130:444 - 60; http://dx.doi.org/10.1016/j.mad.2009.04.005; PMID: 19433103
- Lorenz DR, Cantor CR, Collins JJ. A network biology approach to aging in yeast. Proc Natl Acad Sci USA 2009; 106:1145 - 50; http://dx.doi.org/10.1073/pnas.0812551106; PMID: 19164565
- Simkó GI, Gyurkó D, Veres DV, Nánási T, Csermely P. Network strategies to understand the aging process and help age-related drug design. Genome Med 2009; 1:90; http://dx.doi.org/10.1186/gm90; PMID: 19804610
- Borklu Yucel E, Ulgen KO. A network-based approach on elucidating the multi-faceted nature of chronological aging in S. cerevisiae.. PLoS ONE 2011; 6:e29284; http://dx.doi.org/10.1371/journal.pone.0029284; PMID: 22216232
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, et al. Rates of behavior and aging specified by mitochondrial function during development. Science 2002; 298:2398 - 401; http://dx.doi.org/10.1126/science.1077780; PMID: 12471266
- Rea SL, Ventura N, Johnson TE. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans.. PLoS Biol 2007; 5:e259; http://dx.doi.org/10.1371/journal.pbio.0050259; PMID: 17914900
- Butler JA, Ventura N, Johnson TE, Rea SL. Long-lived mitochondrial (Mit) mutants of Caenorhabditis elegans utilize a novel metabolism. FASEB J 2010; 24:4977 - 88; http://dx.doi.org/10.1096/fj.10-162941; PMID: 20732954
- Gallo M, Park D, Riddle DL. Increased longevity of some C. elegans mitochondrial mutants explained by activation of an alternative energy-producing pathway. Mech Ageing Dev 2011; 132:515 - 8; http://dx.doi.org/10.1016/j.mad.2011.08.004; PMID: 21884719
- Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 2011; 144:79 - 91; http://dx.doi.org/10.1016/j.cell.2010.12.016; PMID: 21215371
- Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans.. Science 2002; 298:830 - 4; http://dx.doi.org/10.1126/science.1074240; PMID: 12399591
- Panowski SH, Dillin A. Signals of youth: endocrine regulation of aging in Caenorhabditis elegans.. Trends Endocrinol Metab 2009; 20:259 - 64; http://dx.doi.org/10.1016/j.tem.2009.03.006; PMID: 19646896
- Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans.. Nature 2007; 447:550 - 5; http://dx.doi.org/10.1038/nature05837; PMID: 17476212
- Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 1982; 215:1415 - 8; http://dx.doi.org/10.1126/science.7063854; PMID: 7063854
- Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol 1985; 40:657 - 70; PMID: 4056321
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev 2005; 126:913 - 22; http://dx.doi.org/10.1016/j.mad.2005.03.012; PMID: 15885745
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009; 460:392 - 5; PMID: 19587680
- Goldberg AA, Richard VR, Kyryakov P, Bourque SD, Beach A, Burstein MT, et al. Chemical genetic screen identifies lithocholic acid as an anti-aging compound that extends yeast chronological life span in a TOR-independent manner, by modulating housekeeping longevity assurance processes. Aging (Albany NY) 2010; 2:393 - 414; PMID: 20622262
- Kaeberlein M. Lessons on longevity from budding yeast. Nature 2010; 464:513 - 9; http://dx.doi.org/10.1038/nature08981; PMID: 20336133
- Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging (Albany NY) 2009; 1:357 - 62; PMID: 20157523
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol 2010; 176:2092 - 7; http://dx.doi.org/10.2353/ajpath.2010.091050; PMID: 20363920
- Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011; 3:148 - 57; PMID: 21386129
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 2011; 10:4230 - 6; http://dx.doi.org/10.4161/cc.10.24.18486; PMID: 22107964
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 2011; 66:191 - 201; http://dx.doi.org/10.1093/gerona/glq178; PMID: 20974732
- Guarente LP, Partridge L, Wallace DC, eds. Molecular Biology of Aging. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2008:610 pages.
- Fannjiang Y, Cheng WC, Lee SJ, Qi B, Pevsner J, McCaffery JM, et al. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev 2004; 18:2785 - 97; http://dx.doi.org/10.1101/gad.1247904; PMID: 15520274
- Pozniakovsky AI, Knorre DA, Markova OV, Hyman AA, Skulachev VP, Severin FF. Role of mitochondria in the pheromone- and amiodarone-induced programmed death of yeast. J Cell Biol 2005; 168:257 - 69; http://dx.doi.org/10.1083/jcb.200408145; PMID: 15657396
- Pereira C, Silva RD, Saraiva L, Johansson B, Sousa MJ, Côrte-Real M. Mitochondria-dependent apoptosis in yeast. Biochim Biophys Acta 2008; 1783; 1286 - 302; PMID: 18406358
- Carmona-Gutierrez D, Eisenberg T, Büttner S, Meisinger C, Kroemer G, Madeo F. Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ 2010; 17:763 - 73; http://dx.doi.org/10.1038/cdd.2009.219; PMID: 20075938
- Wissing S, Ludovico P, Herker E, Büttner S, Engelhardt SM, Decker T, et al. An AIF orthologue regulates apoptosis in yeast. J Cell Biol 2004; 166:969 - 74; http://dx.doi.org/10.1083/jcb.200404138; PMID: 15381687
- Büttner S, Eisenberg T, Carmona-Gutierrez D, Ruli D, Knauer H, Ruckenstuhl C, et al. Endonuclease G regulates budding yeast life and death. Mol Cell 2007; 25:233 - 46; http://dx.doi.org/10.1016/j.molcel.2006.12.021; PMID: 17244531
- Ludovico P, Rodrigues F, Almeida A, Silva MT, Barrientos A, Côrte-Real M. Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae.. Mol Biol Cell 2002; 13:2598 - 606; http://dx.doi.org/10.1091/mbc.E01-12-0161; PMID: 12181332
- Pereira C, Camougrand N, Manon S, Sousa MJ, Côrte-Real M. ADP/ATP carrier is required for mitochondrial outer membrane permeabilization and cytochrome c release in yeast apoptosis. Mol Microbiol 2007; 66:571 - 82; http://dx.doi.org/10.1111/j.1365-2958.2007.05926.x; PMID: 17822411
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 2008; 9:231 - 41; http://dx.doi.org/10.1038/nrm2312; PMID: 18073771
- Ludovico P, Rodrigues F, Almeida A, Silva MT, Barrientos A, Côrte-Real M. Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae.. Mol Biol Cell 2002; 13:2598 - 606; http://dx.doi.org/10.1091/mbc.E01-12-0161; PMID: 12181332
- Silva RD, Sotoca R, Johansson B, Ludovico P, Sansonetty F, Silva MT, et al. Hyperosmotic stress induces metacaspase- and mitochondria-dependent apoptosis in Saccharomyces cerevisiae.. Mol Microbiol 2005; 58:824 - 34; http://dx.doi.org/10.1111/j.1365-2958.2005.04868.x; PMID: 16238630
- Yang H, Ren Q, Zhang Z. Cleavage of Mcd1 by caspase-like protease Esp1 promotes apoptosis in budding yeast. Mol Biol Cell 2008; 19:2127 - 34; http://dx.doi.org/10.1091/mbc.E07-11-1113; PMID: 18321989
- Madeo F, Herker E, Maldener C, Wissing S, Lächelt S, Herlan M, et al. A caspase-related protease regulates apoptosis in yeast. Mol Cell 2002; 9:911 - 7; http://dx.doi.org/10.1016/S1097-2765(02)00501-4; PMID: 11983181
- Fabrizio P, Battistella L, Vardavas R, Gattazzo C, Liou LL, Diaspro A, et al. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae.. J Cell Biol 2004; 166:1055 - 67; http://dx.doi.org/10.1083/jcb.200404002; PMID: 15452146
- Herker E, Jungwirth H, Lehmann KA, Maldener C, Fröhlich KU, Wissing S, et al. Chronological aging leads to apoptosis in yeast. J Cell Biol 2004; 164:501 - 7; http://dx.doi.org/10.1083/jcb.200310014; PMID: 14970189
- Mazzoni C, Herker E, Palermo V, Jungwirth H, Eisenberg T, Madeo F, et al. Yeast caspase 1 links messenger RNA stability to apoptosis in yeast. EMBO Rep 2005; 6:1076 - 81; http://dx.doi.org/10.1038/sj.embor.7400514; PMID: 16170310
- Allen C, Büttner S, Aragon AD, Thomas JA, Meirelles O, Jaetao JE, et al. Isolation of quiescent and nonquiescent cells from yeast stationary-phase cultures. J Cell Biol 2006; 174:89 - 100; http://dx.doi.org/10.1083/jcb.200604072; PMID: 16818721
- Li W, Sun L, Liang Q, Wang J, Mo W, Zhou B. Yeast AMID homologue Ndi1p displays respiration-restricted apoptotic activity and is involved in chronological aging. Mol Biol Cell 2006; 17:1802 - 11; http://dx.doi.org/10.1091/mbc.E05-04-0333; PMID: 16436509
- Aragon AD, Rodriguez AL, Meirelles O, Roy S, Davidson GS, Tapia PH, et al. Characterization of differentiated quiescent and nonquiescent cells in yeast stationary-phase cultures. Mol Biol Cell 2008; 19:1271 - 8; http://dx.doi.org/10.1091/mbc.E07-07-0666; PMID: 18199684
- Fabrizio P, Longo VD. Chronological aging-induced apoptosis in yeast. Biochim Biophys Acta 2008; 1783; 1280 - 5; PMID: 18445486
- Laun P, Heeren G, Rinnerthaler M, Rid R, Kössler S, Koller L, et al. Senescence and apoptosis in yeast mother cell-specific aging and in higher cells: a short review. Biochim Biophys Acta 2008; 1783; 1328 - 34; PMID: 18342634
- Büttner S, Eisenberg T, Herker E, Carmona-Gutierrez D, Kroemer G, Madeo F. Why yeast cells can undergo apoptosis: death in times of peace, love, and war. J Cell Biol 2006; 175:521 - 5; http://dx.doi.org/10.1083/jcb.200608098; PMID: 17101700
- Goldberg AA, Bourque SD, Kyryakov P, Boukh-Viner T, Gregg C, Beach A, et al. A novel function of lipid droplets in regulating longevity. Biochem Soc Trans 2009; 37:1050 - 5; http://dx.doi.org/10.1042/BST0371050; PMID: 19754450
- Beach A, Titorenko VI. In search of housekeeping pathways that regulate longevity. Cell Cycle 2011; 10:3042 - 4; http://dx.doi.org/10.4161/cc.10.18.16947; PMID: 21862878
- Titorenko VI, Terlecky SR. Peroxisome metabolism and cellular aging. Traffic 2011; 12:252 - 9; http://dx.doi.org/10.1111/j.1600-0854.2010.01144.x; PMID: 21083858
- Bitterman KJ, Medvedik O, Sinclair DA. Longevity regulation in Saccharomyces cerevisiae: linking metabolism, genome stability, and heterochromatin. Microbiol Mol Biol Rev 2003; 67:376 - 99; http://dx.doi.org/10.1128/MMBR.67.3.376-399.2003; PMID: 12966141
- Pang CY, Ma YS, Wei YU. MtDNA mutations, functional decline and turnover of mitochondria in aging. Front Biosci 2008; 13:3661 - 75; http://dx.doi.org/10.2741/2957; PMID: 18508463
- Sinclair DA, Oberdoerffer P. The ageing epigenome: damaged beyond repair?. Ageing Res Rev 2009; 8:189 - 98; http://dx.doi.org/10.1016/j.arr.2009.04.004; PMID: 19439199
- D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 2007; 8:813 - 24; http://dx.doi.org/10.1038/nrm2256; PMID: 17848967
- Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals?. Nat Rev Mol Cell Biol 2007; 8:722 - 8; http://dx.doi.org/10.1038/nrm2240; PMID: 17700625
- Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell 2007; 26:1 - 14; http://dx.doi.org/10.1016/j.molcel.2007.03.016; PMID: 17434122
- Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger. Cell Metab 2008; 7:200 - 3; http://dx.doi.org/10.1016/j.cmet.2008.01.001; PMID: 18316025
- Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, et al. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet 2008; 4:e13; http://dx.doi.org/10.1371/journal.pgen.0040013; PMID: 18225956
- Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab 2007; 5:265 - 77; http://dx.doi.org/10.1016/j.cmet.2007.02.009; PMID: 17403371
- Pan Y, Shadel GS. Extension of chronological life span by reduced TOR signaling requires down-regulation of Sch9p and involves increased mitochondrial OXPHOS complex density. Aging (Albany NY) 2009; 1:131 - 45; PMID: 20157595
- Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab 2011; 13:668 - 78; http://dx.doi.org/10.1016/j.cmet.2011.03.018; PMID: 21641548
- Ocampo A, Liu J, Schroeder EA, Shadel GS, Barrientos A. Mitochondrial respiratory thresholds regulate yeast chronological life span and its extension by caloric restriction. Cell Metab 2012; 16:55 - 67; http://dx.doi.org/10.1016/j.cmet.2012.05.013; PMID: 22768839
- Kyryakov P, Beach A, Richard VR, Burstein MT, Leonov A, Levy S, et al. Caloric restriction extends yeast chronological lifespan by altering a pattern of age-related changes in trehalose concentration. Front Physiol 2012; 3:256; http://dx.doi.org/10.3389/fphys.2012.00256; PMID: 22783207
- Longo VD, Mitteldorf J, Skulachev VP. Programmed and altruistic ageing. Nat Rev Genet 2005; 6:866 - 72; http://dx.doi.org/10.1038/nrg1706; PMID: 16304601
- Skulachev VP, Longo VD. Aging as a mitochondria-mediated atavistic program: can aging be switched off?. Ann N Y Acad Sci 2005; 1057:145 - 64; http://dx.doi.org/10.1196/annals.1356.009; PMID: 16399892
- Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle 2006; 5:2087 - 102; http://dx.doi.org/10.4161/cc.5.18.3288; PMID: 17012837
- Blagosklonny MV. Paradoxes of aging. Cell Cycle 2007; 6:2997 - 3003; http://dx.doi.org/10.4161/cc.6.24.5124; PMID: 18156807
- Blagosklonny MV. Program-like aging and mitochondria: instead of random damage by free radicals. J Cell Biochem 2007; 102:1389 - 99; http://dx.doi.org/10.1002/jcb.21602; PMID: 17975792
- Blagosklonny MV. TOR-driven aging: speeding car without brakes. Cell Cycle 2009; 8:4055 - 9; http://dx.doi.org/10.4161/cc.8.24.10310; PMID: 19923900
- Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle 2010; 9:3151 - 6; http://dx.doi.org/10.4161/cc.9.16.13120; PMID: 20724817