Abstract
Comment on: Li F, et al. Proc Natl Acad Sci USA 2012; 109:10966-71.
The tumor necrosis factor receptor (TNFR) superfamily members are type I transmembrane proteins widely expressed on both normal and malignant tissues and control essential biological processes including cell apoptosis, activation and proliferation.Citation1 A number of TNFR signaling pathways have been reported to be beneficial in immune and antitumor responses. For example, CD40-mediated immunostimulatory effects and DR5-mediated, direct apoptotic effects display potent antitumor activity through distinct mechanisms. Agonistic antibodies targeting TNFRs have therefore been extensively investigated as a therapeutic approach to trigger TNFR signaling pathways in antitumor responses. Despite their impressive performance in animal models, clinical results of these agonistic anti-TNFR antibodies appear to be disappointing. Our recent studies on the role of the Fc for agonistic anti-CD40Citation2 and anti-DR5Citation3 antibodies suggest an explanation for this discrepancy between the pre-clinical animal studies and clinical application of these agonistic antibodies. It is now apparent that agonistic anti-TNFR antibodies display a general requirement for FcγRIIB co-engagement for their in vivo activity, and the selection of the appropriate Fc was not taken into consideration in the advancement of therapeutic versions of these antibodies into the clinic.
Both mice and humans have several activating Fcγ receptors (FcγRs) and one inhibitory FcγR, FcγRIIB.Citation4 While antibodies that mediate antitumor effects through antibody-dependent, cell-mediated cytotoxicity (ADCC) clearly require activating FcγR engagement, whether and how FcγRs contribute to the in vivo activities of agonistic antibodies is less clear. Although early studies had suggested that both activating and inhibitory FcγRs might function to augment the activity of agonistic antibodies under in vitro conditions to activate the signaling pathways of targeted membrane molecules, in vivo studies of the effect of FcγRs on the function of agonistic anti-TNFR antibodies did not support such a mechanism. Recently, we and others reported that FcγRIIB plays a unique role among all the FcγRs in the in vivo function of agonistic anti-CD40 antibodies, where FcγRIIB was found to be both necessary and sufficient for the immunostimulatory and antitumor activities of agonistic anti-CD40 antibodies.Citation2,Citation5 CD40 belongs to one of the two broad categories of TNFRs, the TNF receptor-associated factors (TRAF) pathway, based on the signaling pathway used by its cytoplasmic domains. The other major category of signaling pathway is triggered by TNFRs known as death receptors, such as DR5, that signal through Fas-associated protein with death domain (FADD) adaptor molecules. We have recently found that FcγRIIB is also required for the apoptotic and antitumor activities of agonistic anti-DR5 antibodies, and the presence of activating FcγRs appears to reduce their activities by competing with FcγRIIB.Citation3 These studies suggest that FcγRIIB co-engagement is a general requirement of agonistic antibodies targeting both TNFR subfamilies.
Additional evidence for a general requirement for FcγRIIB co-engagement also comes from in vivo studies of some other agonistic anti-TNFR antibodies in animal models with targeted FcγR mutations. Agonistic anti-Fas antibodies were shown to specifically require FcγRIIB, not activating FcγRs in vivo in a hepatotoxicity model,Citation6 and Wilson et al. showed that human DR5-specific antibodies exert antitumor activities in mice lacking activating FcγRI and FcγRIII, but display reduced antitumor activities in mice lacking FcγRIIB.Citation7 In an anaplastic large-cell lymphoma (ALCL) xenograft model, the antitumor activities of anti-human CD30 antibodies were shown to be activating FcγR-independent.Citation8
Furthermore, the comparison between agonistic anti-TNFR antibodies with Fcs that preferentially bind to activating or inhibitory FcγRs, respectively, in in vivo studies also supports the notion of a general requirement for FcγRIIB and not activating FcγR co-engagement. Both mouse IgG1 and rat IgG2a Fcs preferentially bind to mouse FcγRIIB, whereas mouse IgG2a and rat IgG2b Fcs preferentially bind to activating FcγRs (ref. Citation4, and F.L. and J.V.R., unpublished data). Chuntharapai et al. reported an isotype-dependent inhibition of tumor growth using anti-human DR4 antibodies in the human Colo 205 colon carcinoma xenograft model, where anti-human DR4 antibodies with mouse IgG1 Fcs were much more effective than the ones with mouse IgG2a Fcs.Citation9 In another study, Sakanishi, et al. showed that among several rat anti-mouse CD27 antibody clones, a non-depleting clone with rat IgG2a Fc was more effective than a depleting clone with rat IgG2b Fc in the antitumor responses against both CD27+ and CD27- EL4 syngeneic tumors.Citation10 Interestingly, and also consistently, as far as we know, there seems to be no reported good agonistic anti-TNFR antibodies with mouse IgG2a or rat IgG2b Fcs in in vivo studies.
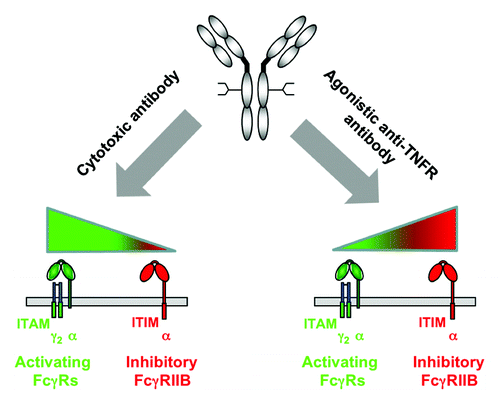
Although the exact mode of action that underlines the requirement for FcγRIIB co-engagement remains to be determined, the requirement for FcγRIIB co-engagement can be exploited to make more potent agonistic antibodies. Our studies of agonistic anti-CD40 and anti-DR5 antibodies have demonstrated that in both cases, increasing FcγRIIB binding can enhance the in vivo activities of these antibodies.Citation2,Citation3 FcγRIIB-targeted Fc engineering might be generalized to other agonistic anti-TNFR antibodies and further the translation of TNFR biology into therapeutic applications ().
Figure 1. Antibody engineering approaches to enhance cytotoxic and agonistic anti-TNFR antibodies. Shown is the differential contribution of activating and inhibitory FcγRs to the in vivo activities of cytotoxic and agonistic anti-TNFR antibodies. Based on this model, antibody engineering approaches to enhance cytotoxic antibody function should focus on increased binding to activating FcγRs, whereas the activity of agonistic anti-TNFR antibodies may be optimized by a selective binding to the inhibitory FcγRIIB. Activating FcγRs containing immunoreceptor tyrosine-based activation motifs (ITAM) are shown in green; inhibitory FcγRIIB containing an immunoreceptor tyrosine-based inhibition motif (ITIM) is shown in red.
References
- Aggarwal BB. Nat Rev Immunol 2003; 3:745 - 56; http://dx.doi.org/10.1038/nri1184; PMID: 12949498
- Li F, et al. Science 2011; 333:1030 - 4; http://dx.doi.org/10.1126/science.1206954; PMID: 21852502
- Li F, et al. Proc Natl Acad Sci USA 2012; 109:10966 - 71; http://dx.doi.org/10.1073/pnas.1208698109; PMID: 22723355
- Nimmerjahn F, et al. Nat Rev Immunol 2008; 8:34 - 47; http://dx.doi.org/10.1038/nri2206; PMID: 18064051
- White AL, et al. J Immunol 2011; 187:1754 - 63; http://dx.doi.org/10.4049/jimmunol.1101135; PMID: 21742972
- Xu Y, et al. J Immunol 2003; 171:562 - 8; PMID: 12847219
- Wilson NS, et al. Cancer Cell 2011; 19:101 - 13; http://dx.doi.org/10.1016/j.ccr.2010.11.012; PMID: 21251615
- Zhang M, et al. Blood 2006; 108:705 - 10; http://dx.doi.org/10.1182/blood-2005-11-4607; PMID: 16551968
- Chuntharapai A, et al. J Immunol 2001; 166:4891 - 8; PMID: 11290766
- Sakanishi T, et al. Biochem Biophys Res Commun 2010; 393:829 - 35; http://dx.doi.org/10.1016/j.bbrc.2010.02.092; PMID: 20171165