Abstract
Comment on: Lv L, et al. Cell Cycle 2012; 11:2864-75.
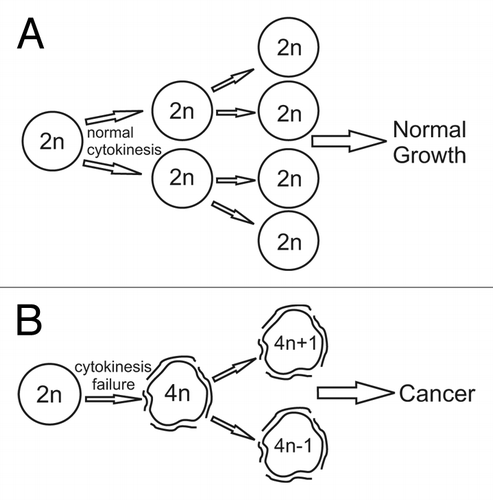
Aneuploidy is ubiquitous in cancer and has been causally linked to tumorigenesis.Citation1 Although many decades of intense research have provided invaluable information on the causes and consequences of aneuploidy (reviewed in ref. Citation1), many questions still remain. For example, it is still unclear whether the degree of aneuploidy observed in cancer is the result of multiple subsequent losses/gains of one to few chromosomes, or if it is the result of an initial tetraploidization event followed by chromosome loss/gain events.Citation2 Furthermore, although tetraploidy has been observed in certain pre-cancerous lesions, thus making it a potential tumor promoter, it is not clear how tetraploidy affects aneuploidization and tumorigenesis.Citation1 In a recent study, Lv, et al. began to provide answers to these questions by characterizing the karyotypes and cell division defects of mouse ovarian surface epithelial (MOSE) cells, which spontaneously transform after in vitro passaging over time.Citation3 Lv, et, al. showed that the rate of cytokinesis failure in MOSE cells increases with passage number, yielding more tetraploid cells.Citation3 These tetraploid cells continue to proliferate, but they display higher rates of chromosome mis-segregation compared to their diploid progenitors, leading to the generation of numerous aneuploid daughter cells.Citation3 Recent studies showing that clustering of supernumerary centrosomes is a major cause of chromosome mis-segregationCitation4-Citation6 support the findings of Lv, et al. To investigate how the observed cellular events affect tumorigenesis, Lv, et al. injected late-passage (p35) MOSE cells into syngenic mice and saw tumors in 100% of mice.Citation3 The rapid transformation of MOSE cells with the concurrent generation of aneuploidy underscores the importance of aneuploidy in tumorigenesis. Indeed, it may be more appropriate to describe the transformation of MOSE cells as aneuploidy-induced instead of spontaneous, since passaging of chromosomally stable immortalized cells for a similar amount of time does not result in tumorigenesis.Citation6 Similar to the study by Lv, et al., previous work in mouse mammary epithelial cells showed the same sequence of events: cytokinesis failure-tetraploidy-aneuploidy-tumorigenesis.Citation7 However, unlike the work by Lv, et al., this sequence of events in mouse mammary epithelial cells relied upon p53 mutation or loss.Citation7 It is unclear whether or not this is true for the MOSE cells used by Lv, et al. Preliminary microarray data indicate a decrease in p53 expression levels in MOSE cells during progression (Schmelz, personal communication), but loss of p53 in MOSE cells has not been reported to date. Thus, it is unlikely that p53 was spontaneously lost in the Lv, et al. and other independent studies using MOSE cells,Citation8 adding to the controversy of a “tetraploidy checkpoint.”Citation9 While more work may be needed to understand whether p53 plays any role in modulating a response to tetraploidy and in the MOSE cancer progression model, it is clear from the work by Lv, et al. that tetraploidy can occur early in tumorigenesis, act as an intermediate for aneuploidization and, ultimately, cause cancer ().Citation3
Figure 1. Tetraploidy can act as an intermediate for aneuploidization and cause cancer. Cells undergoing normal cytokinesis (A) maintain a stable karyotype and exhibit normal growth. Cytokinesis failure (B) generates tetraploid cells whose karyotype becomes unstable due to high rates of chromosome segregation errors. Such instability leads to high rates of aneuploidy and tumorigenesis.
References
- Nicholson JM, et al. In: David G, ed. Advances in cancer research: Academic Press, 2011:43-75.
- Cimini D.. Biochim Biophys Acta 2008; 1786; 32 - 40
- Lv L, et al. Cell Cycle 2012; 11:2864 - 75; http://dx.doi.org/10.4161/cc.21196; PMID: 22801546
- Silkworth WT, et al. PLoS One 2009; 4:e6564; http://dx.doi.org/10.1371/journal.pone.0006564; PMID: 19668340
- Ganem NJ, et al. Nature 2009; 460:278 - 82; http://dx.doi.org/10.1038/nature08136; PMID: 19506557
- Jiang XR, et al. Nat Genet 1999; 21:111 - 4; http://dx.doi.org/10.1038/5056; PMID: 9916802
- Fujiwara T, et al. Nature 2005; 437:1043 - 7; http://dx.doi.org/10.1038/nature04217; PMID: 16222300
- Roberts PC, et al. Neoplasia 2005; 7:944 - 56; http://dx.doi.org/10.1593/neo.05358; PMID: 16242077
- Wong C, et al. BMC Cell Biol 2005; 6:6; http://dx.doi.org/10.1186/1471-2121-6-6; PMID: 15713235