Cell growth and proliferation are fundamental processes in living organisms and are dynamically controlled by environmental cues. TOR complex 1 (TORC1) is a central protein kinase involved in the regulation of cell growth in response to a wide variety of cellular states and is the target of the immunosuppressant and anticancer drug rapamycin. In the budding yeast Saccharomyces cerevisiae, TORC1 contains the catalytic subunit Tor1 (or Tor2) and accessory subunits, including Kog1, Lst8 and Tco89. TORC1 signaling is primarily regulated by nitrogen/amino acid availability.Citation1 In addition, recent studies revealed that TORC1 activity is also repressed by a variety of cellular stresses, including glucose starvation, heat, oxidants and high osmolarity, under all of which cell growth is compromised.Citation1
How individual environmental cues influence TORC1 activity has been largely unknown.Citation1 Recent identification of new regulators of TORC1 greatly advances our understanding of its regulation via input from nitrogen/amino acids. Small GTPases, Gtr1 and Gtr2, are key players in the regulation of TORC1 in response to amino acid availability by directly associating with TORC1 and recruiting it to the vicinity of the vacuolar membrane, where Gtr1 and Gtr2 reside.Citation2 Both Gtr1 and Gtr2 are included in the vacuolar membrane-associated protein complex, called the EGO complex,Citation2 which functions as a regulator of microautophagy as well as TORC1. These regulators are highly conserved in mammalian cells.Citation3 Thus, the vacuole (the lysosome in mammals) seems to be an important organelle that acts as the site of TORC1 activation. In contrast to TORC1 regulation by nitrogen/amino acids, its regulation under stress conditions has only recently been discovered and is poorly understood. Therefore, it is of great importance to find other unidentified critical factors that function in the regulation of TORC1 to coordinately control cell growth in S. cerevisiae under various physiological conditions. We sought to identify novel regulators of TORC1 signaling to uncover the comprehensive regulatory mechanisms. Our genetic screening using a hyperactive TOR1 allele led to the identification of Pbp1 as a novel negative regulator of TORC1 signaling, revealing an unexpected link between TORC1 and stress granules ().Citation4
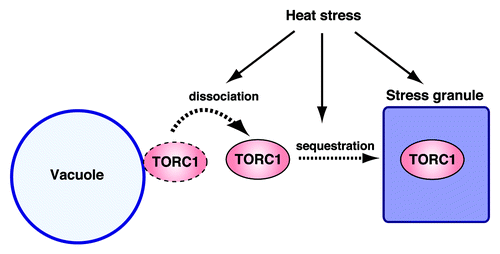
Figure 1. Heat stress triggers TORC1 sequestration into stress granules. TORC1 is normally localized in the vicinity of the vacuolar membrane. Under heat stress, TORC1 dissociates from the vacuolar membrane and relocalizes to the stress granules, a process underpinning the protective response to heat-induced cellular damage.
Pbp1 was identified as a binding protein of Pab1, the poly(A)-binding protein regulating mRNA metabolism and translation. During specific stress conditions, such as heat, glucose starvation or high ethanol concentration, messenger ribonucleoprotein (mRNP), including Pab1 and Pbp1, is dynamically remodeled, thereby leading to the formation of cytoplasmic mRNP granules called stress granules.Citation5-Citation7 The physiological significance of the formation of these granules in S. cerevisiae is largely unknown, but is thought to regulate mRNA fates under stress conditions. A previous reportCitation8 stating that Pbp1 overexpression causes ectopic formation of stress granules under normal growth conditions prompted us to analyze the potential regulation of TORC1 by stress granules. Our subsequent analyses revealed that Pbp1 overexpression leads to the downregulation of TORC1 signaling by promoting the association of TORC1 with the aberrant stress granules.
One key finding is that TORC1 dissociates from the vacuolar membrane and is sequestered into stress granules during heat stress (). This TORC1 sequestration into heat-induced stress granules controls the activation status of TORC1 signaling during the recovery phase after heat stress. Once sequestered into the stress granules, TORC1 is maintained in an inactive state. Subsequently, stress granules govern TORC1 reactivation by controlling the restoration of TORC1 to the vacuolar membrane through stress granule disassembly. Our findings further suggest that the control of TORC1 activity via stress granules protects cells from heat-induced DNA damage. Thus, stress granules appear to act as a sensor of cellular heat damage and coordinately make the decision between resuming cell growth and repairing heat damage, highlighting the physiological significance of stress granule formation during heat stress.
Our new findings reveal the close connection between TORC1 and stress granules, but also raise many unresolved questions. What are the factors escorting TORC1 to heat-induced stress granules? Identification of such factors as well as a master regulator of stress granule formation in yeast will greatly expand our understanding of stress granule-mediated protection against a variety of stresses. Because one major function of TORC1 is promotion of mRNA translation, it is possible that TORC1 sequestration into stress granules not only makes TORC1 generally inactive, but also specifically functions to enhance the translation of particular mRNAs required for protection against heat stress. Although this work revealed TORC1 relocalization during the recovery phase after heat stress, it remains unknown how TORC1 initially dissociates from the vacuolar membrane. Under heat stress conditions, the components of the EGO complex, including Gtr1/Gtr2, are still associated with the vacuolar membrane (unpublished results); therefore, it is likely that heat stress triggers the dissociation of Gtr1/Gtr2 and TORC1. Thus, the initial inactivation of TORC1 signaling by heat stress is independent of its sequestration into stress granules and may involve the Rho1-mediated inactivation of TORC1.Citation9 Finally, as the structure, function and regulation of TORC1 is highly conserved throughout evolution, it is of considerable interest to determine whether stress granule-TORC1 regulation also operates in mammalian cells under conditions promoting stress granule formation such as exposure to toxic reagents, high temperature and viral infection.Citation5,Citation10
References
- Loewith R, et al. Genetics 2011; 189:1177 - 201; http://dx.doi.org/10.1534/genetics.111.133363; PMID: 22174183
- Binda M, et al. Mol Cell 2009; 35:563 - 73; http://dx.doi.org/10.1016/j.molcel.2009.06.033; PMID: 19748353
- Sancak Y, et al. Cell 2010; 141:290 - 303; http://dx.doi.org/10.1016/j.cell.2010.02.024; PMID: 20381137
- Takahara T, et al. Mol Cell 2012; 47:242 - 52; http://dx.doi.org/10.1016/j.molcel.2012.05.019; PMID: 22727621
- Buchan JR, et al. Mol Cell 2009; 36:932 - 41; http://dx.doi.org/10.1016/j.molcel.2009.11.020; PMID: 20064460
- Grousl T, et al. J Cell Sci 2009; 122:2078 - 88; http://dx.doi.org/10.1242/jcs.045104; PMID: 19470581
- Kato K, et al. Yeast 2011; 28:339 - 47; http://dx.doi.org/10.1002/yea.1842; PMID: 21341306
- Swisher KD, et al. PLoS One 2010; 5:e10006; http://dx.doi.org/10.1371/journal.pone.0010006; PMID: 20368989
- Yan G, et al. Mol Cell 2012; 45:743 - 53; http://dx.doi.org/10.1016/j.molcel.2012.01.028; PMID: 22445487
- Kedersha N, et al. Prog Mol Biol Transl Sci 2009; 90:155 - 85; http://dx.doi.org/10.1016/S1877-1173(09)90004-7; PMID: 20374741