Pluripotency, the capacity of a cell to give rise to differentiated derivatives that represent each of the three primary germ layers, belongs to the cells that are located within the inner cell mass (ICM) of the developing blastocyst. Functional studies have identified a group of transcription factors, the pluripotency transcription factors that affect the pluripotent capacity.Citation1 Within this group, the transcription factors Oct4 (Pou5f1), Nanog and Sox2 are crucial for the efficient maintenance of pluripotent cell identity.Citation1 During mouse development, the specification of pluripotent cell identity requires the embryonic genome to express Oct4 and Nanog,Citation1 but not Sox2,Citation2 perhaps owing to the presence of long-lived maternal Sox2 protein. Pluripotency transcription factors regulate stem cell pluripotency and differentiation via the colocalization and the cooperation with each other, polycomb repressive complexes (PRC) and microRNAs in the transcriptional and epigenetic regulation of key stem cell genes.Citation3
Stem cell is a specific cell population with the abilities of self-renewal and multipotent differentiation. According to the origin and potentiality of the cells, mammalian stem cells could be classified into two groups: one is embryonic stem cells (ESCs), which are isolated from ICM of blastocysts; the other is adult stem cells, such as hematopoietic stem cells, neural stem cells and mesenchymal stem cells (MSCs), which are found in adult tissues. In addition, it has been demonstrated that somatic cell can be reprogrammed to pluripotent-like stem cells, otherwise known as induced pluripotent stem-like cells (iPS) by overexpressing specific pluripotency transcription factors, including Oct4, Sox2, c-Myc and Klf4, or Oct4, Nanog, Sox2 and Lin28.Citation4
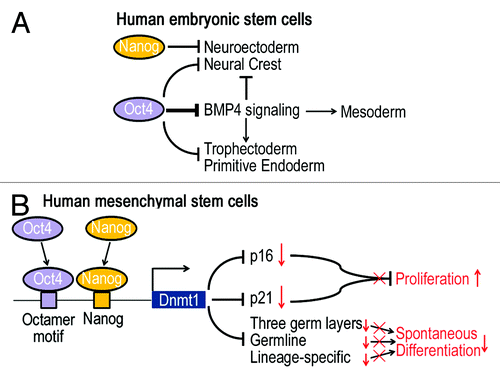
Expression of pluripotency transcription factors is restricted to pluripotent cells and is downregulated upon differentiation.Citation1 In mouse ESCs, downregulation of Oct4 induces the differentiation into trophoblast lineage, while reduction in Nanog induces differentiation into extra-embryonic endoderm.Citation5 However, the roles of these pluripotency transcription factors in maintaining ESC self-renewal and differentiation are not the same across human and mouse. Z. Wang and his colleagues investigated the roles of Oct4, Nanog and Sox2 in human ESCs using the Lentiviral-knockdown system.Citation6 They identified that high levels of Oct4 and Nanog, rather than Sox2, are indispensable for maintaining self-renewal of human ESCs. Moreover, they found Oct4 directly inhibits the BMP4 signaling pathway, which activates mesoderm and extraembryonic ectoderm/endoderm differentiation, while Nanog acts as a repressor of neural crest and neuroectoderm lineage ().
Figure 1. Schematization of the potential signaling pathways that Oct4 and Nanog mediate to regulate self-renewal and differentiation of human ESCs and MSCs. (A) In human ESCs, Oct4 inhibits the BMP4 signaling pathway, which activates mesoderm and extraembryonic ectoderm/endoderm differentiation, while Nanog acts as a repressor of neural crest and neuroectoderm lineage. (B) In human MSCs, Oct4 and Nanog cooperatively induce Dnmt1 expression, which leads to DNA methylation and suppression of p16, p21, developmental markers and lineage genes, thereby promoting proliferation and maintaining undifferentiated states.
MSCs have somewhat similar stem cell properties to ESCs with regards to their maintenance and differentiation potential. Many studies also demonstrated the expression of pluripotency transcription factors in MSCs and their involvement in regulation of stem cell properties.Citation7 However, the expression and the roles of these pluripotent genes in adult stem cells have been controversial, since knockout of Oct4 did not affect the ability of MSCs in colony formation and differentiation into bone, fat and cartilage.Citation8 To clarify the roles of pluripotency transcription factors in MSC maintenance, we first demonstrate that MSCs expanded under normoxic conditions underwent significant changes in cell proliferation rate, differentiation potential and expression of developmental markers and tissue-specific genes and also decreased in the expression of Oct4 and Nanog.Citation9,Citation10 Our recent studies further demonstrated that the expression of Oct4 and Nanog was higher in MSCs at early passage (E), in hypoxic culture (H) and with p21 knockdown (p21KD) compared with MSCs at late passage (L), in normoxic culture (N) and with scrambled shRNA-overexpression (Scr), respectively.Citation10 The expression of Oct4 and Nanog was not only localized in the nucleus, but associated with a less methylated pattern in the CpG regions of their promoters. Knockdown of Oct4 and Nanog in E, H and p21KD MSCs reduced cell proliferation rate and differentiation potential but induced the expression of higher levels of various developmental markers and tissue-specific genes, while overexpression of Oct4 and Nanog in L, N and Scr MSCs increased cell proliferation rate as well as differentiation potential and inhibited spontaneous differentiation. These data suggest that Oct4 and Nanog are not only essential for the maintenance of pluripotency in ESCs, but are also essential for maintaining MSC properties.
Our studies further demonstrated that Oct4 and Nanog directly bind to the promoter of Dnmt1 and enhance its expression, which, in turn, downregulates the expression of cell cycle regulators such as p16 and p21 as well as development and lineage genes.Citation10 Meanwhile, the binding sites for Oct4 and Nanog were only 38 bases apart, suggesting that Oct4 and Nanog in MSCs, like in ESCs, work together to regulate downstream genes.Citation3 Moreover, MSCs, when treated with inhibitor of DNA methylation or transfected with shRNA against Dnmt1, had decreased proliferation rate and differentiation potential, but increased expression of genes associated with senescence and developmental regulators. These data suggest MSCs like ESCs undergo changes in methylation of pluripotency genes upon expansion in culture, and Oct4 and Nanog cooperatively induce Dnmt1 expression to regulate the proliferative and undifferentiated states of MSCs ().
Since our study is focused on the roles of Oct4 and Nanog in maintaining MSC properties, we have not checked whether the same phenomenon occurred in other stem cells or somatic cells. However, it would be interesting to know whether the regulation of Dnmt1 by Oct4 and Nanog is also found in other adult stem cells or pluripotent stem cells. Notably, overexpression of Dnmt1 also rescues somatic cells from serum starvation-induced upregulation of p16 and p21 as well as cell cycle arrest.Citation10 This finding argues that Oct4 and Nanog control their downstream genes through indirectly controlling their expression by binding to the Dnmt1 promoter.
References
- Chambers I, et al. Development 2009; 136:2311 - 22; http://dx.doi.org/10.1242/dev.024398; PMID: 19542351
- Avilion AA, et al. Genes Dev 2003; 17:126 - 40; http://dx.doi.org/10.1101/gad.224503; PMID: 12514105
- Kashyap V, et al. Stem Cells Dev 2009; 18:1093 - 108; http://dx.doi.org/10.1089/scd.2009.0113; PMID: 19480567
- Loh YH, et al. Cell Cycle 2008; 7:885 - 91; http://dx.doi.org/10.4161/cc.7.7.5636; PMID: 18414030
- Hough SR, et al. Stem Cells 2006; 24:1467 - 75; http://dx.doi.org/10.1634/stemcells.2005-0475; PMID: 16456133
- Wang Z, et al. Cell Stem Cell 2012; 10:440 - 54; http://dx.doi.org/10.1016/j.stem.2012.02.016; PMID: 22482508
- Greco SJ, et al. Stem Cells 2007; 25:3143 - 54; http://dx.doi.org/10.1634/stemcells.2007-0351; PMID: 17761754
- Lengner CJ, et al. Cell Stem Cell 2007; 1:403 - 15; http://dx.doi.org/10.1016/j.stem.2007.07.020; PMID: 18159219
- Tsai CC, et al. Blood 2011; 117:459 - 69; http://dx.doi.org/10.1182/blood-2010-05-287508; PMID: 20952688
- Tsai CC, et al. Mol Cell 2012; 47:169 - 82; http://dx.doi.org/10.1016/j.molcel.2012.06.020; PMID: 22795133