p53 is a tumor-suppressor protein regulating cell cycle progression, apoptosis, senescence, autophagy, metabolism, stem cell differentiation and inflammatory responses. Its function, as a genome guardian sensing DNA damage and acting primarily as a transcription factor to control gene activity, is often deregulated in cancer cells by genetic mutation or guilt association with unintended viral and cellular factors that alter p53’s gene targets, compartmentalization, protein stability or posttranslational modification state. An important function of p53 in mitotic progression that has been previously noted—abnormal centrosome amplification, leading to chromosome segregation defects—was first observed in p53-null mouse embryonic fibroblasts.Citation1 However, the molecular mechanism underlying p53-regulated centrosome formation remains elusive. In a recent issue of Cell Cycle, Wu et al. identified Aurora A kinase normally associating with centrosomes as a functional target of p53 occurring at both transcriptional and post-transcriptional levels.Citation2 The abundance of Aurora A transcripts in wild-type p53-containing cells could be regulated by RNA polymerase II-dependent transcription via the p53-pRb-E2F3 pathway (, pathway 1). When p53 is downregulated or inactivated by oncogenic signaling, transcription from the cell cycle inhibitor p21 gene is suppressed, leading to upregulated cyclin-dependent kinase 2 (CDK2) activity that phosphorylates pRb and releases E2F3 transcription factor, which, in turn, activates Aurora A gene transcription. Interestingly, reduced p53 level also diminishes transcription from another p53 target gene encoding the Fbw7α component of an E3 ubiquitin ligase that degrades Aurora A, resulting in an increased amount of Aurora A protein at the post-transcriptional level (, pathway 2). A significant increase of Aurora A RNA and protein by concurrently inducing p53-pRb-E2F3 and p53-Fbw7α pathways causes mitotic checkpoint deregulation, abnormal centrosome amplification, chromosome segregation defects, aneuploidy and eventually carcinogenesis (, pathway 3).
Figure 1. p53-Aurora A mitotic feedback loop regulating p53 and Aurora A function. Distinct pathways modulating p53 and Aurora A activity at the transcriptional (Trx) or post-transcriptional (Post-Trx) level are depicted by thick lines, numbered one to seven, with P indicating phosphorylation (PO4) and up and down red arrows reflecting upregulated and downregulated protein or RNA amounts, respectively.
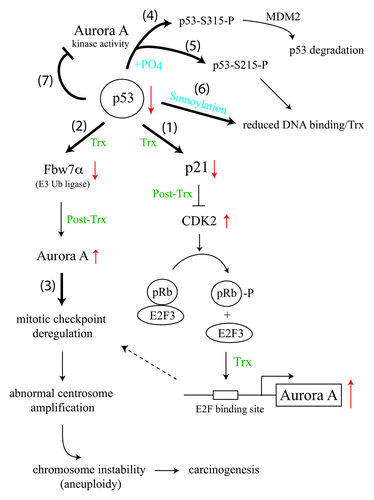
While p53 inversely regulates the protein level of Aurora A by both transcriptional and post-transcriptional pathways, the kinase activity of Aurora A also provides a feedback mechanism to regulate the protein stability and DNA-binding activity of p53 by phosphorylating p53 at serine 315, triggering MDM2-induced p53 degradation (, pathway 4) or by phosphorylating p53 at serine 215, blocking its DNA-binding activity (, pathway 5).Citation3,Citation4 Inactivation of p53 by Aurora A-mediated phosphorylation at the residue corresponding to serine 215 of human p53 is essential for maintaining self-renewal and pluripotency of mouse embryonic stem cells,Citation5 a scenario analogous to cancer cell growth triggered by overexpression of Aurora A and loss of p53 function. Phosphorylation-blocked p53 binding to DNA at serine 215 by Aurora A downregulates p53 target gene transcription, similar to the effect of PIAS family proteins-mediated sumoylation at lysine 386 of p53, which also inhibits the DNA-binding activity of p53 and suppresses its transcriptional activity (, pathway 6).Citation6 Intriguingly, p53 can reciprocally inhibit the kinase activity of Aurora A by interacting with the N-terminal region of Aurora A to block the enzymatic activity of Aurora A residing in its C-terminal region,Citation7 perhaps via allosteric regulation (, pathway 7). Clearly, a fine balance between counteracting Aurora A and p53 function is critical for driving mitotic progression in normal cells. Paradoxically, a complete loss of p53 protein, as seen in p53-null mice, leads to frequent deletion or downregulation of Aurora A loci,Citation8 further highlighting the essence of maintaining a functional p53-Aurora A feedback loop in mitotic cell cycle control. Considering that Aurora B kinase can also phosphorylate p53 and lead to its degradation,Citation9 some extent of functional redundancy likely exists in Aurora family proteins as well, even though their subcellular localizations appear different throughout cell cycle progression.
References
- Fukasawa K, et al. Science 1996; 271:1744 - 7; http://dx.doi.org/10.1126/science.271.5256.1744; PMID: 8596939
- Wu CC, et al. Cell Cycle 2012; 11; http://dx.doi.org/10.4161/cc.21732; PMID: 22894933
- Katayama H, et al. Nat Genet 2004; 36:55 - 62; http://dx.doi.org/10.1038/ng1279; PMID: 14702041
- Liu Q, et al. J Biol Chem 2004; 279:52175 - 82; http://dx.doi.org/10.1074/jbc.M406802200; PMID: 15469940
- Lee DF, et al. Cell Stem Cell 2012; 11:179 - 94; http://dx.doi.org/10.1016/j.stem.2012.05.020; PMID: 22862944
- Wu SY, et al. EMBO J 2009; 28:1246 - 59; http://dx.doi.org/10.1038/emboj.2009.83; PMID: 19339993
- Chen SS, et al. EMBO J 2002; 21:4491 - 9; http://dx.doi.org/10.1093/emboj/cdf409; PMID: 12198151
- Mao JH, et al. Cancer Cell 2007; 11:161 - 73; http://dx.doi.org/10.1016/j.ccr.2006.11.025; PMID: 17292827
- Gully CP, et al. Proc Natl Acad Sci USA 2012; 109:E1513 - 22; http://dx.doi.org/10.1073/pnas.1110287109; PMID: 22611192