Abstract
A hallmark of cancer is reactivation/alteration of pathways that control cellular differentiation during developmental processes. Evidence indicates that WNT, Notch, BMP and Hedgehog pathways have a role in normal epithelial cell differentiation, and that alterations in these pathways accompany establishment of the tumorigenic state. Interestingly, there is recent evidence that these pathways are intertwined at the molecular level, and these nodes of intersection may provide opportunities for effective targeted therapies. This review will highlight the role of the WNT, Notch, BMP and Hedgehog pathways in colon cancer.
Introduction
Alterations in genes that control developmental processes during embryogenesis and organogenesis are recognized as hallmarks of cancer. Pathways such as wingless-related integration site (WNT), Hedgehog (HH), Notch and bone morphogenic protein (BMP) are well-characterized in the developing embryo for establishing cell position, body pattern segmentation, polarity and cell fate decisions.Citation1 It is not surprising that several of these signaling pathways are altered in oncogenic processes.Citation1 This was perhaps first recognized in the hematopoietic system, in which overexpression of various Hox genes is associated with a variety of leukemias.Citation2
Similarly, epithelial cancers also exhibit alterations in genetic pathways more commonly associated with embryogenesis.Citation1 This review will highlight four of these pathways, WNT, Notch, Hedgehog and BMP, and will discuss their role in colon cancer and their possible utility as therapeutic targets.
Colon Cancer
With an average of 50,000 deaths per year, colorectal cancer has emerged as the second leading cause of cancer death in the United States and worldwide.Citation3-Citation5 Early diagnosis and surgical intervention, along with combination chemotherapy, has resulted in improved outcomes.Citation6 However, there are few effective strategies to treat colon cancer once first-line approaches have been exhausted. Improved understanding of the cellular basis for colon cancer and the role that signaling pathways such as WNT (wingless-related integration site), BMP (bone morphogenic protein), Notch and HH (hedgehog) have in the establishment and maintenance of the tumorigenic state will be critical for the development of novel therapeutics.Citation7-Citation9 The advent of small-molecule inhibitors for targeting these pathways and their success in other diseases, either as single agents or in combination therapy, provides a rationale for exploiting these pathways as potential targets in the treatment of colon cancer.Citation10
The unit of structure in the normal colon is the crypt of Lieberkuhn, which is composed of colon stem cells, transit amplifying cells and terminally differentiated goblet cells, enterocytes and endocrine cells.Citation11 Each normal crypt is comprised of about 2,000 cells.Citation12 Similar to the crypt of the small intestine, less differentiated cells reside in the bottom, and terminally differentiated cells reside near the top. Thus, there is a developmental hierarchy from bottom (undifferentiated) to top (differentiated) in the colon crypts, which consists of a stem/progenitor compartment, a proliferative zone and a differentiated compartment (). These cells continuously cycle from undifferentiated in the bottom of the crypt through the terminally differentiated cells at the top.Citation11,Citation12 This process is controlled by the colon stems cells and the microenvironment. The colon epithelium is replaced nearly every 5 d.
Figure 1. Signaling in the colon crypt. Illustration indicating the overall structure of the human colon crypt and the gradient of WNT, Notch, BMP and HH signaling. Also indicated are the stem, proliferative and differentiative zones of the crypt, illustrating how colon epithelial cells differentiate from bottom of the crypt to top.
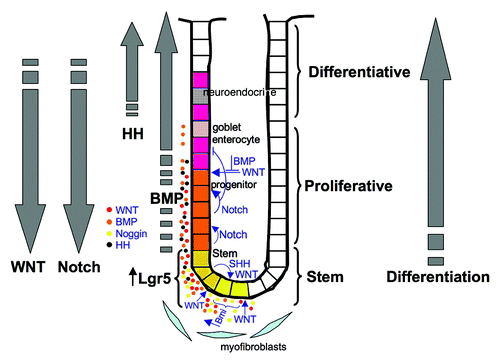
The architectural structure of the colon is reflected by a gradient of WNT, HH, BMP and Notch signaling ().Citation1,Citation8 Starting at the base of the crypt unit, Notch signaling is highest in the stem cell compartment and decreases as cells move upwards through the proliferative areas and into the differentiative areas. WNT signaling is similar, with the highest levels of expression being in the earliest stages of the proliferative compartment and tailing off in the differentiative compartment.Citation13 BMP signaling is active in the differentiated compartment, and despite the presence of BMP protein, it is relatively inactive in early compartments in the base of the crypt due to the presence of the BMP inhibitor Noggin.Citation14,Citation15 HH expression primarily occurs in the differentiated compartment. Thus, these gradients of developmentally regulated signaling pathways serve to establish the pattern of stem cell/self-renewal, proliferation and differentiation that comprise the colon architecture.
The normal colon has two distinct pools of stem cells, which together make up the total population of 16 stem cells.Citation11,Citation12,Citation16 The contribution of each pool to the total is not known. The first stem cell pool is localized in the crypt base and can be characterized by high LGR5 expression and is largely comprised of a proliferating population.Citation17,Citation18 The next pool is nearby in the +4 position of the colon base (four cells away from the base of the crypt) and consists of relatively quiescent or dormant cells. This second pool exhibits high expression of BMI-1 and telomerase reverse transcriptase (TERT).Citation11
Nearly 70% of colon cancers arise from adenomatous polyps; masses of cells that emerge from the intestinal mucusa and project into the colon lumen.Citation6,Citation19 Of the three broad types of polyps, juvenile (harmatomous), hyperplastic and adenomatous, only the adenomatous polyps are associated with the emergence of sporadic colon adenocarcinoma.Citation6 Not all adenomatous polyps will give rise to colon tumors, and these can be further subdivided based on gross or histologic appearance. Visualization and classification of polyps are the basis for early detection and prevention of colon cancer through colonoscopy or sigmoidoscopy.Citation6
Adenocarcinomas of the colon can take up to 10 y to completely emerge. This sequence was first proposed by Fearon and Volgelstein,Citation20 building upon the Knudson two-hit hypothesis,Citation21,Citation22 to be a stepwise accumulation of specific genetic mutation. Activating mutations in the Ras oncogene, and inactivating mutations in the APC and/or p53 tumor suppressors are frequently found in the majority of sporadic colon tumors.Citation19,Citation23-Citation25 In part, due to a better understanding of the function of APC in WNT signaling, data has emerged that alteration of developmentally regulated pathways, which function in normal colon formation, have a role in colon carcinogeneis.Citation8,Citation26
Pathways
WNT
In the canonical WNT pathway, WNT ligand binds to the Frizzled receptor and the LRP5/6 coreceptor.Citation26,Citation27 This results in stabilization of the Disheveled gene, which leads to cytoplasmic accumulation and subsequent nuclear translocation of β-catenin. It is involvement of β-catenin that defines canonical WNT signaling as compared with alternative WNT/Frizzled signaling pathways.Citation26,Citation28
The pool of β-catenin is regulated through phosphorylation by glycogen synthase kinase 3 (GSK3), in which phosphorylated β-catenin is targeted for proteosomal degradation.Citation26 Together, with Axin and APC, phosphorylated β-catenin is shunted to the proteasome. In the presence of WNT ligand, GSK3 activity is inhibited by cytoplasmic Disheveled, thus stabilizing β-catenin for translocation to nucleus. Stabilized, free β-catenin that translocates to the nucleus associates with TCF/LEF to regulate transcription. Noncanonical WNT signaling also uses the Frizzled receptor, but involves an increasing list of co-receptors that includes Cripto and Ror2.Citation29 This alternative pathway typically involves PLC and PKC signaling.Citation30 In colon cancer, WNT target genes include c-mycCitation31 TCF-1,Citation32 LEF1,Citation33,Citation34 c-jun,Citation35 MMP-7,Citation36,Citation37 CD44,Citation38 VEGF,Citation39 Jagged1Citation40 and BMP4.Citation41 Many of these gene targets are likely tissue and/or cell type-specific, but Axin2 is widely used as a general indicator of WNT pathway activation.Citation42 For a comprehensive list of WNT target genes, see the WNT homepage at www.wnt.stanford.edu.
Mutations in the WNT pathway cause colon cancer through constitutive activation of the nuclear β-catenin/TCF transcription factor complex.Citation13,Citation43 The most well-documented WNT pathway mutation in colon cancer is loss of the APC tumor suppressor gene.Citation11,Citation13,Citation26,Citation43 APC normally functions as part of a complex that also contains GSK-3β and Axin. This complex destabilizes β-catenin through its phosporylation by GSK-3β and its subsequent degradation by the ubiquitin/proteasome pathway. Nearly 90% of sporadic colon cancers contain loss of function at both alleles of APC, resulting in constitutive stabilization of β-catenin and activation of WNT pathway genes, namely TCF, which are required for colon crypt maintenance.Citation43 This results in inappropriate proliferation that presents as colon polyps. Interestingly, point mutations in β-catenin have been identified in the approximately 15% of sporadic colon cancers that bear wild-type alleles of APC.Citation44 These mutations in β-catenin render it insensitive to de-stabilization by the Axin/GSK-3β/APC complex and result in constitutive WNT signaling.Citation43,Citation44 However, mutations in both APC and β-catenin have yet to be identified within the same tumor.Citation43
Improved strategies for high-throughput screening have driven the development of WNT inhibitors.Citation45-Citation47 Clinicaltrials.gov lists compounds that are undergoing clinical testing for WNT pathway inhibition, including LGK974 (Novartis, NCT01351103), CWP232201 (JW Pharmaceutical, NCT01398462) and PRI724 (Prism BioLab, NCT01302405). Despite the fact that nearly 80% of colon cancers bear activating mutations in the WNT pathway, the PRI724 compound is the only one of these in which colorectal cancer is included in the study. In addition to synthetic inhibitors, monoclonal antibodies and natural products are also being tested as WNT inhibitors. OTSA011 is a mAb against Frizzled homolog 10 (FZD10) that is currently being tested (Centre Leon Berard/Oncotherapy Science, NCT01469975). An interesting trial that examined resveratrol, a plant-derived natural phenol, in colon cancer, was recently concluded.Citation48 Reseveratrol has been implicated in colon cancer prevention.Citation49 Based on reports that some of resveratrol’s effects could be attributed to WNT pathway inhibition, a small cohort of colorectal and normal patients was given low-dose, plant-purified resveratrol (NCT00256334).Citation48 However, no effect on WNT signaling was observed in colorectal cancer, although WNT was inhibited in normal colon mucosa.
Nonsteroidal anti-inflamatory drugs have been shown to have some effect in inhibiting WNT signaling. This would include aspirin, indomethacin and celecoxib.Citation50,Citation51 These Cox-2 inihibitors are thought to inhibit WNT by suppressing prostaglandin E2 (PGE2) production, since PGE2 has been shown to promote WNT signaling. A connection between WNT and mTOR led to the observation that the mTOR inhibitor everolimus could reduce the number of colon polyps and cancer mortality in a mouse model.Citation52
Interestingly, inhibition of the WNT pathway may not be the only approach. Activation of non-canonical WNT signaling by HDAC inhibitors has been reported to inhibit the growth of colon cancer cells in the presence APC mutations.Citation53 Given the importance of the WNT pathway in several diseases, in addition to colon cancer, the development of effective inhibitory strategies should remain a priority.
Notch signaling
The Notch signaling pathway in humans consists of four receptors, Notch-1, -2, -3, -4 and at least five ligands, Jagged-1, Jagged-2, Delta-1, Delta-3 and Delta-4.Citation54 The Notch pathway is highly conserved, with homologs in species ranging from worms through Man.Citation55 In the canonical Notch pathway, ligand interaction with receptor results in a cascade of proteolytic cleavages mediated first by a metalloprotease, and second by a γ-secretase activity that is made up of at least the presenilin, nicastrin and Aph proteins.Citation54 These cleavage steps result in release of a constitutively active intracytoplasmic Notch (ICN) fragment that is then translocated to the nucleus, where it associates with CBF-1 and MAML-1 as part of a larger transcription complex.Citation56 The net effect of ICN is to switch transcriptional complexes of CBF-1 from repression to activation.Citation57 The precise signaling differences between different Notch-receptor and Notch-ligand pairing is not well-understood. While they all seem to go through the same pathway, there is evidence that different receptor-ligand parings yield distinct biological outcomes.Citation58 In addition, glycosylation of the Notch receptors adds an additional layer of complexity when considering biochemical and biologic outcomes from specific Notch receptor-Notch ligand pairings.Citation58-Citation60 Notch signaling is terminated by CDK8-mediated phosphorylation of a PEST domain on the ICN. This then targets ICN for proteosomal degredation and allows the cells to be responsive to new Notch signals.Citation54-Citation56
Notch-1, the most widely studied Notch receptor, was first identified from a t(7;9) translocation in a subset of T-cell acute leukemia (T-ALL), which fuses the cytoplasmic portion of Notch-1 to the T-cell receptor β-locus, resulting in constitutive activation of Notch-1.Citation61 Subsequent studies confirmed that constitutive Notch-1 was likely the causative agent of this rare subset of T-ALL.Citation62 More recent work has demonstrated that nearly 80% of all T-ALL bear activating mutations in the Notch-1 receptor, demonstrating a role for Notch-1 as an oncogene within the T-lineage.Citation63 On the other hand, Notch-1 functions as a tumor suppressor in chronic myelomonocytic leukemia (CMML), in which inactivating mutations of Notch-1 have been identified.Citation64 Within the B-lineage, enforced expression of constitutively active Notch results in the death of B-lineage acute leukemia (B-ALL) cell lines, indicating a tumor-suppressive role for Notch.Citation65 Thus, even within the hematopoietic system, Notch-1 can function as either to repress or promote tumorigenesis, dependent upon cell type.
In solid tumors, the role for Notch-1 is less well-characterized, but evidence for a dual oncogene/tumor suppressor role has been reported.Citation66 Oncogenic Notch has been reported in breast epithelial tumors and is thought to have a role in tamoxifen resistance.Citation67,Citation68 On the other hand, some studies have reported a potential role for Notch as a tumor suppressor. Inactivating mutations in Notch-1 have been reported in squamous cell carcinoma, suggesting that the role Notch signaling may have in tumor biology is likely cell- and tissue type-specific.Citation69-Citation71 The best characterized role for Notch as a tumor suppressor is in skin keratinocytes.Citation72 The presence of functional Notch is required to protect keratinocytes from chemical or UV-mediated transformation.Citation73,Citation74
The most well-documented molecular target of Notch-1 signaling is Hes-1.Citation75 Transcriptional activation of Hes or HEY family genes appears universal in nearly all Notch systems studied.Citation75 Other Notch targets appear to be more cell type-specific. c-Myc, p27Kip1, p57, Akt, p53 and PTEN are just a few Notch target genes that have also been implicated in tumorigenesis, independently of Notch.Citation66,Citation74
The role of Notch signaling in normal intestinal development has been well-documented and is the subject of several excellent reviews,Citation1,Citation11,Citation12,Citation76-Citation78 and hence will not be extensively discussed here. However, the contribution of Notch signaling to colon cancer is not as well-characterized. Expression of the Notch-1 receptor and its target Hes-1 was reported to increase with increasing tumor grade in a gene array analysis consisting of 10% colon mucosa, 15% colonic polyps, 55% primary colon cancers and 13% liver metastasis.Citation79 Notch-2 levels were not increased, nor were levels of Jagged-1 or Delta-3 ligand. Expression of the Notch antagonist, Numb, was decreased in advanced colon cancers. These data suggest a general activation of Notch-1 signaling in colon cancer. Another group used in situ hybridization to examine the Notch pathway, and concluded that Notch signaling was active in colon tumors, but did not find a correlation between Hes-1 gene expression and survival among colon cancer patients.Citation80
Interestingly, investigators have reported that treatment of colon cancer cell lines with oxaliplatin, 5-FU or SN-38 (irinotecan) upregulates the γ-secretase complex and results in increased levels of cleaved activated Notch. They went on to demonstrate that treatment with a γ-secretase inhibitor would render the cell lines more sensitive to chemotherapeutic treatment.Citation79 In a recent clinical trial, however, the γ-secretase inhibitor RO4929097 was tested as single agent in metastatic colorectal cancer with little to no effect.Citation81 There are currently two additional trials testing the efficacy of RO4929097 in colorectal cancer in combination with other chemotherapeutic drugs (NCT01198535 and NCT01270438).
Other recent research used a colorectal cancer explant model to evaluate the effectiveness of the γ-secreatese inhibitor PF-03084014 in combination with irinotecan.Citation82 In this model, tumor fragments isolated from patient material were explanted into nude mice, which were then treated with PF-03084014, irinotecan or both. PF-03084014 plus irinotecan was more effective than γ-secretase inhibitor or irinotecan administered as single agents. This same group also demonstrated that for tumors with elevated Notch expression, PF-03084014 plus irinotecan treatment resulted in reduced tumor recurrence. The bulk of these effects were in ALDH+ tumor cells, which is a subpopulation enriched for CIC (cancer initiating cell) activity.Citation12,Citation82 Presently, there is a phase one clinical trial with PF-03084014 (NCT00878189, Pfizer).
BMP
Bone morphogenetic proteins (BMP), first identified for their role in controlling bone formation, are members of the TGF β superfamily.Citation83 BMPs bind to the BMP receptors, BMPRI or BMPRII. Both of these are serine-threonine kinase receptors. BMP binding to BMPRII results in phosphorylation of BMPRI, which subsequently phosphorylates Smad1, Smad5 and Smad8. These then associate with Smad4, resulting in activation and nuclear localization.Citation84 Extracellular molecules such as Noggin can regulate BMP signaling by sequestering BMP away from the BMPRI and BMPRII.Citation84
In colon cancer, mutations in Smad4 or BMPRI have been shown to be responsible for juvenile polyposis.Citation14 In sporadic colon cancer, loss of phosphorylation of Smad1, Smad5 and Smad8 has been observed in 70% of cancers.Citation85 Loss of Smad4 or loss or BMPRII is the likely mechanistic basis for loss of BMP signaling in sporadic colon cancers. However, because studies have indicated that loss of BMP signaling in sporadic colon cancers correlates with tumor grade, it is likely that this is not an initiating event (as it is in juvenile polyposis), but rather contributes to tumor progression.Citation14
It is estimated that nearly 80% of colon cancers bear mutations in the TGF β family signaling pathway.Citation14 Either BMPRI or BMPRII have been reported to be mutated in more than 70% of cases.Citation86 About 20–30% of colorectal cancer cases bear mutations in Smad4.Citation87,Citation88 There is increasing evidence in sporadic colon cancers (as compared with JP) that mutations affecting BMP signaling corroborate with activated WNT to drive colon cancers, particularly in later stages.Citation14 It has been reported that Smad4 levels are predictive of outcome/prognosis in stage II colorectal cancers. Those with low Smad4 levels only had a median survival of 1.7 y compared with greater than 9 y for patients with a high Smad4 level.Citation89,Citation90 These clinical observations are consistent with a recent report that signaling through Smad4 can inhibit colon cancer progression by reducing the expression of β-catenin. BMP signaling has also been reported to promote the growth of colon carcinomas.Citation91
Hedgehog
The Hedgehog (HH) pathway derives its unusual name from the phenotype of hedgehog loss in Drosophila; larvae take on a curled, bristly appearance that may remind some of a hedgehog.Citation1 In humans, there are three HH proteins, Sonic HH, Indian HH and Desert HH. Sonic HH is the most well-studied isoform.Citation1,Citation92 HH is synthesized as a 45 kDa precursor that is self-cleaved into a C- and N-terminal peptides. The role of the C-terminal peptide is unknown, but the N terminal forms the active HH ligand.Citation93 Diffusible HH can bind to its receptor, Patched, which then de-represses the membrane-bound protein Smoothened (Smo). This last step results in activation and release of Gli transcription factors and their subsequent nuclear translocation.Citation1 Vertebrates have three Gli proteins. Gli1 will result in activation of HH target genes, while Gli3 is a repressor of signaling. Gli2 serves a dual role, with both repressive and activator functions.Citation1
Genes regulated by HH signaling include Myc, Bcl-2 and the Notch ligand, Jagged2.Citation94 Also induced by HH signaling are the stem cell-associated proteins LGR5, CD133 and CD44, as well as transcription factors that regulate epithelial to mesenchymal transition (EMT) such as Snail, Slug and Twist.Citation94
Mutations that result in activation of HH signaling are the driver mutations in basal cell carcinomas, for which there are now targeted therapies.Citation95 Pharmaceuticals targeting the HH pathway have also been used in some instances of multiple myeloma (MM) and chronic myeloid leukemia (CML).Citation96
Evidence from mouse models indicates that HH may cooperate with activated WNT to drive lethality in colon cells.Citation97 This suggests that HH inhibitors may be an interesting target to consider in colon cancer. The best known example of a HH inhibitor is the plant-derived steroid cyclopamine.Citation1 This alkaloid binds to Smo, resulting in its inactivation. Cycloplanine has been shown to have anti-tumorigenic activity in a variety of laboratory oncogenic settings. With the recent success of HH pathway inhibitors in the treatment of basal cell carcinoma [e.g., visodegib/erivedge (Roche/Genentech)], HH has moved more to the forefront of a potential targeted therapy in cancer.Citation96 Clinicaltrials.gov lists 34 trials recruiting for seven different inhibitors. Only one, LEQ-506 Novartis, lists colon cancer among the tumors to be tested. HH inhibitors are also being tested in combination with Notch inhibitors in advanced breast cancer and sarcoma.Citation8
Communication Between Pathways
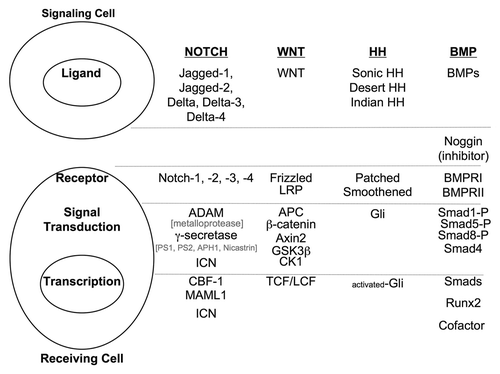
summarizes the linear process of signaling for the WNT, Notch, BMP and HH pathways. However, there is an increasing body of evidence from a variety of tissues that these developmental pathways exhibit cross-talk or share molecular points (nodes) of intersection.Citation1,Citation29 The first report of such cross-talk was in fruit flies, in which it was shown that wingless (the fly homolog of WNT) signaling could be regulated by Notch via a mechanism in which disheveled would bind to the cytoplasmic tail of Notch.Citation98 WNT signals can also control Gli3 from the HH pathway.Citation99 HH can antagonize WNT signaling in the colon.Citation100,Citation101 Likewise, HH has been reported to control the expression of the Notch ligand Jagged2, whereas WNT/β-catenin can control Jagged1.Citation102,Citation103 Hes-1 can be activated by both Notch and HH signaling.Citation104-Citation106 BMP and WNT appear to be interconnected via the PI3k/Akt pathway.Citation107 TGF β/Smad signaling promotes EMT through WNT, Ras, HH and Notch.Citation108 In APC mutant mice, Notch signaling is required for the development of colon polyps and subsequent cancer.Citation97,Citation109 Thus, there is interplay between these pathways, and alterations in one could have potential effects on others.
Figure 2. Summary of Notch, WNT HH and BMP signaling pathways. Illustrated are the major components of each of the Notch, WNT, HH and BMP pathways, starting with ligands expressed by the signaling cell, receptors expressed on the receiving cell and cytoplasmic signaling intermediates and transcriptional effectors. In this illustration, signal transduction for each pathway travels from the top of the figure to the bottom.
Kwon et al. recently reported physical interaction between β-catenin and the cytoplasmic tail of membrane bound Notch.Citation110 Only the active pool of β-catenin protein was capable of binding to Notch. They provided evidence that this interaction would result in degradation of β-catenin protein and is one mechanism by which WNT signaling is modulated.
In addition to cross-talk, these various developmental pathways can also have an impact on cell signaling pathways such as PI3K/Akt and Ras/Raf/Mek/Erk.Citation111-Citation114 In one recent paper, it was demonstrated that Ras signaling is enhanced through increased stabilization of Ras in colon cancers that bear mutated APC (in the WNT pathway). The authors presented data that stabilization of Ras was controlled by altered WNT activity regulating recruitment of Ras to the proteosome.Citation115 Given that Ras inhibitors have performed poorly in clinical trials; this paper emphasizes the need for examining and identifying molecular connections between pathways. Interactions between WNT and Ras have also been reported in lung cancer.Citation116 Hedgehog and Ras have been reported to be interconnected in colon cancer.Citation117 Other work has implicated interactions between PTEN/PI3K/Akt signaling and BMP in colon cancers.Citation112,Citation118 Insulin-like growth factor-1 (IGF1) has been reported to promote the growth of colon cancer cells via a β-catenin-dependent mechanism.Citation119 Targeting IGF1R with a specific monoclonal antibody or inhibition of Akt reduced colon tumor growth.Citation120 Connections between HH and p53 have also been proposed, further illustrating the complex interconnectivity between signaling pathways.Citation120,Citation121
Summary
WNT, Notch, BMP and HH represent fundamental pathways that regulate development. In the past decade, a role for these pathways in oncogenesis has emerged: driving the development of targeted therapies. Research suggests that these pathways do not act in isolation, but are interconnected such that alterations in one lead to alterations in another. Understanding how signaling pathways are interconnected is critical to the development of successful targeted therapies. By focusing on the molecular points of intersection, it may be possible to develop more efficient strategies for therapy.
Recent studies into tumor heterogeneity illustrate the complexity of cancer at the molecular level.Citation122-Citation125 These recent studies have indicated that the accumulation of mutations contributing to tumor heterogeneity may not be mere bystanders, but may have an active role in establishing a unique biologic phenotype that make each cancer individual.Citation126 By understanding the interplay between pathways such as WNT, Notch, HH and BMP we can hope to develop global strategies to effectively target a broad array of cancers.
References
- Geissler K, Zach O. Pathways involved in Drosophila and human cancer development: the Notch, Hedgehog, Wingless, Runt, and Trithorax pathway. Ann Hematol 2012; 91:645 - 69; http://dx.doi.org/10.1007/s00277-012-1435-0; PMID: 22418742
- Look AT. Oncogenic transcription factors in the human acute leukemias. Science 1997; 278:1059 - 64; http://dx.doi.org/10.1126/science.278.5340.1059; PMID: 9353180
- American Cancer Society, Colorectal Cancer Facts & Figures 2011-2013; http://www.cancer.org/Research/CancerFactsFigures/ColorectalCancerFactsFigures/colorectal-cancer-facts-figures-2011-2013-page.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69 - 90; http://dx.doi.org/10.3322/caac.20107; PMID: 21296855
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011; 61:212 - 36; http://dx.doi.org/10.3322/caac.20121; PMID: 21685461
- Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet 2010; 375:1030 - 47; http://dx.doi.org/10.1016/S0140-6736(10)60353-4; PMID: 20304247
- Takebe N, Ivy SP. Controversies in cancer stem cells: targeting embryonic signaling pathways. Clin Cancer Res 2010; 16:3106 - 12; http://dx.doi.org/10.1158/1078-0432.CCR-09-2934; PMID: 20530695
- Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol 2011; 8:97 - 106; http://dx.doi.org/10.1038/nrclinonc.2010.196; PMID: 21151206
- van Es JH, Clevers H. Notch and Wnt inhibitors as potential new drugs for intestinal neoplastic disease. Trends Mol Med 2005; 11:496 - 502; http://dx.doi.org/10.1016/j.molmed.2005.09.008; PMID: 16214417
- Lea MA. Recently identified and potential targets for colon cancer treatment. Future Oncol 2010; 6:993 - 1002; http://dx.doi.org/10.2217/fon.10.53; PMID: 20528236
- Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature 2011; 474:318 - 26; http://dx.doi.org/10.1038/nature10212; PMID: 21677748
- Vaiopoulos AG, Kostakis ID, Koutsilieris M, Papvassiliou AG. Concise review: colorectal stem cells. Stem Cells 2012; 30:363 - 71; http://dx.doi.org/10.1002/stem.1031; PMID: 22232074
- Burgess AW, Faux MC, Layton MJ, Ramsay RG. Wnt signaling and colon tumorigenesis--a view from the periphery. Exp Cell Res 2011; 317:2748 - 58; http://dx.doi.org/10.1016/j.yexcr.2011.08.010; PMID: 21884696
- Hardwick JC, Kodach LL, Offerhaus GJ, van den Brink GR. Bone morphogenetic protein signalling in colorectal cancer. Nat Rev Cancer 2008; 8:806 - 12; http://dx.doi.org/10.1038/nrc2467; PMID: 18756288
- Kosinski C, Li VSW, Chan ASY, Zhang J, Ho C, Tsui WY, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci USA 2007; 104:15418 - 23; http://dx.doi.org/10.1073/pnas.0707210104; PMID: 17881565
- Botchkina G. Colon cancer stem cells - From basic to clinical application. [epub ahead of print] Cancer Lett 2012; http://dx.doi.org/10.1016/j.canlet.2012.04.006; PMID: 22537805
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449:1003 - 7; http://dx.doi.org/10.1038/nature06196; PMID: 17934449
- Kemper K, Grandela C, Medema JP. Molecular identification and targeting of colorectal cancer stem cells. Oncotarget 2010; 1:387 - 95; PMID: 21311095
- Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol 2011; 6:479 - 507; http://dx.doi.org/10.1146/annurev-pathol-011110-130235; PMID: 21090969
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990; 61:759 - 67; http://dx.doi.org/10.1016/0092-8674(90)90186-I; PMID: 2188735
- Knudson AG. Mutation and human cancer. Adv Cancer Res 1973; 17:317 - 52; http://dx.doi.org/10.1016/S0065-230X(08)60534-5
- Knudson AG. Two genetic hits (more or less) to cancer. Nat Rev Cancer 2001; 1:157 - 62; http://dx.doi.org/10.1038/35101031; PMID: 11905807
- Baker DJ, van Deursen JM. Chromosome missegregation causes colon cancer by APC loss of heterozygosity. Cell Cycle 2010; 9:1711 - 6; http://dx.doi.org/10.4161/cc.9.9.11314; PMID: 20404532
- Michor F, Iwasa Y, Rajagopalan H, Lengauer C, Nowak MA. Linear model of colon cancer initiation. Cell Cycle 2004; 3:358 - 62; http://dx.doi.org/10.4161/cc.3.3.690; PMID: 14726709
- Amos-Landgraf JM, Clipson L, Newton MA, Dove WF. The many ways to open the gate to colon cancer. Cell Cycle 2012; 11:1261 - 2; http://dx.doi.org/10.4161/cc.19888; PMID: 22421162
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012; 149:1192 - 205; http://dx.doi.org/10.1016/j.cell.2012.05.012; PMID: 22682243
- Hoffman J, Kuhnert F, Davis CR, Kuo CJ. Wnts as essential growth factors for the adult small intestine and colon. Cell Cycle 2004; 3:554 - 7; http://dx.doi.org/10.4161/cc.3.5.858; PMID: 15044853
- Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J 2012; 31:2670 - 84; http://dx.doi.org/10.1038/emboj.2012.146; PMID: 22617420
- Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem 2011; 112:3491 - 501; http://dx.doi.org/10.1002/jcb.23287; PMID: 21793042
- Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium 2005; 38:439 - 46; http://dx.doi.org/10.1016/j.ceca.2005.06.022; PMID: 16099039
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science 1998; 281:1509 - 12; http://dx.doi.org/10.1126/science.281.5382.1509; PMID: 9727977
- Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R, et al. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science 1999; 285:1923 - 6; http://dx.doi.org/10.1126/science.285.5435.1923; PMID: 10489374
- Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet 2001; 28:53 - 7; http://dx.doi.org/10.1038/ng0501-53; PMID: 11326276
- Mj F, Cheng N, Abott D, Leonticu V, Engelhardt JF. WNT3A/Beta-catenin signaling induces transcription from the LEF-1 promoter. J Biol Chem 2002; 227:33398 - 410
- Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, et al. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci USA 1999; 96:1603 - 8; http://dx.doi.org/10.1073/pnas.96.4.1603; PMID: 9990071
- Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 1999; 155:1033 - 8; http://dx.doi.org/10.1016/S0002-9440(10)65204-2; PMID: 10514384
- Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene 1999; 18:2883 - 91; http://dx.doi.org/10.1038/sj.onc.1202627; PMID: 10362259
- Wielenga VJ, Smits R, Korinek V, Smit L, Kielman M, Fodde R, et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol 1999; 154:515 - 23; http://dx.doi.org/10.1016/S0002-9440(10)65297-2; PMID: 10027409
- Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res 2001; 61:6050 - 4; PMID: 11507052
- Rodilla V, Villanueva A, Obrador-Hevia A, Robert-Moreno A, Fernández-Majada V, Grilli A, et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci USA 2009; 106:6315 - 20; http://dx.doi.org/10.1073/pnas.0813221106; PMID: 19325125
- Kim JS, Crooks H, Dracheva T, Nishanian TG, Singh B, Jen J, et al. Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res 2002; 62:2744 - 8; PMID: 12019147
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 2002; 22:1184 - 93; http://dx.doi.org/10.1128/MCB.22.4.1184-1193.2002; PMID: 11809809
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell 2000; 103:311 - 20; http://dx.doi.org/10.1016/S0092-8674(00)00122-7; PMID: 11057903
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997; 275:1787 - 90; http://dx.doi.org/10.1126/science.275.5307.1787; PMID: 9065402
- Clevers H. Wnt breakers in colon cancer. Cancer Cell 2004; 5:5 - 6; http://dx.doi.org/10.1016/S1535-6108(03)00339-8; PMID: 14749120
- Lepourcelet M, Chen Y-NP, France DS, Wang H, Crews P, Petersen F, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell 2004; 5:91 - 102; http://dx.doi.org/10.1016/S1535-6108(03)00334-9; PMID: 14749129
- Curtin JC, Lorenzi MV. Drug discovery approaches to target Wnt signaling in cancer stem cells. Oncotarget 2010; 1:563 - 77; PMID: 21317452
- Nguyen AV, Martinez M, Stamos MJ, Moyer MP, Planutis K, Hope C, et al. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag Res 2009; 1:25 - 37; PMID: 21188121
- Hofseth LJ. singh UP, Singh NP, Nagarkatti M, Nagarkatti PS. Taming the beast within: resveratrol suppresses colitis and prevents colon cancer. Aging 2012; 2L:183 - 4
- Dihlmann S, Siermann A, von Knebel Doeberitz M. The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene 2001; 20:645 - 53; http://dx.doi.org/10.1038/sj.onc.1204123; PMID: 11313997
- Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000; 342:1946 - 52; http://dx.doi.org/10.1056/NEJM200006293422603; PMID: 10874062
- Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc Natl Acad Sci USA 2008; 105:13544 - 9; http://dx.doi.org/10.1073/pnas.0800041105; PMID: 18768809
- Sikandar S, Dizon D, Shen X, Li Z, Besterman J, Lipkin SM. The class I HDAC inhibitor MGCD0103 induces cell cycle arrest and apoptosis in colon cancer initiating cells by upregulating Dickkopf-1 and non-canonical Wnt signaling. Oncotarget 2010; 1:596 - 605; PMID: 21317455
- Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol 2000; 228:151 - 65; http://dx.doi.org/10.1006/dbio.2000.9960; PMID: 11112321
- Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development 2011; 138:3593 - 612; http://dx.doi.org/10.1242/dev.063610; PMID: 21828089
- Kovall RA. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene 2008; 27:5099 - 109; http://dx.doi.org/10.1038/onc.2008.223; PMID: 18758478
- Lai EC. Keeping a good pathway down: transcriptional repression of Notch pathway target genes by CSL proteins. EMBO Rep 2002; 3:840 - 5; http://dx.doi.org/10.1093/embo-reports/kvf170; PMID: 12223465
- D’Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene 2008; 27:5148 - 67; http://dx.doi.org/10.1038/onc.2008.229; PMID: 18758484
- Haines N, Irvine KD. Glycosylation regulates Notch signalling. Nat Rev Mol Cell Biol 2003; 4:786 - 97; PMID: 14570055
- Stanley P. Regulation of Notch signaling by glycosylation. Curr Opin Struct Biol 2007; 17:530 - 5; http://dx.doi.org/10.1016/j.sbi.2007.09.007; PMID: 17964136
- Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 1991; 66:649 - 61; http://dx.doi.org/10.1016/0092-8674(91)90111-B; PMID: 1831692
- Pear WS, Aster JC, Scott ML, Hasserjian RP, Soffer B, Sklar J, et al. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med 1996; 183:2283 - 91; http://dx.doi.org/10.1084/jem.183.5.2283; PMID: 8642337
- Weng AP, Ferrando AA, Lee W, Morris JP 4th, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 2004; 306:269 - 71; http://dx.doi.org/10.1126/science.1102160; PMID: 15472075
- Klinakis A, Lobry C, Abdel-Wahab O, Oh P, Haeno H, Buonamici S, et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature 2011; 473:230 - 3; http://dx.doi.org/10.1038/nature09999; PMID: 21562564
- Zweidler-McKay PA, He Y, Xu L, Rodriguez CG, Karnell FG, Carpenter AC, et al. Notch signaling is a potent inducer of growth arrest and apoptosis in a wide range of B-cell malignancies. Blood 2005; 106:3898 - 906; http://dx.doi.org/10.1182/blood-2005-01-0355; PMID: 16118316
- Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer 2011; 11:338 - 51; http://dx.doi.org/10.1038/nrc3035; PMID: 21508972
- Pandya K, Meeke K, Clementz AG, Rogowski A, Roberts J, Miele L, et al. Targeting both Notch and ErbB-2 signalling pathways is required for prevention of ErbB-2-positive breast tumour recurrence. Br J Cancer 2011; 105:796 - 806; http://dx.doi.org/10.1038/bjc.2011.321; PMID: 21847123
- Hao L, Rizzo P, Osipo C, Pannuti A, Wyatt D, Cheung LW, et al. Notch-1 activates estrogen receptor-alpha-dependent transcription via IKKalpha in breast cancer cells. Oncogene 2010; 29:201 - 13; http://dx.doi.org/10.1038/onc.2009.323; PMID: 19838210
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011; 333:1157 - 60; http://dx.doi.org/10.1126/science.1208130; PMID: 21798893
- Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011; 333:1154 - 7; http://dx.doi.org/10.1126/science.1206923; PMID: 21798897
- Wang NJ, Sanborn Z, Arnett KL, Bayston LJ, Liao W, Proby CM, et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc Natl Acad Sci USA 2011; 108:17761 - 6; http://dx.doi.org/10.1073/pnas.1114669108; PMID: 22006338
- Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet 2003; 33:416 - 21; http://dx.doi.org/10.1038/ng1099; PMID: 12590261
- Mandinova A, Lefort K, Tommasi di Vignano A, Stonely W, Ostano P, Chiorino G, et al. The FoxO3a gene is a key negative target of canonical Notch signalling in the keratinocyte UVB response. EMBO J 2008; 27:1243 - 54; http://dx.doi.org/10.1038/emboj.2008.45; PMID: 18388864
- Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep 2008; 9:377 - 83; http://dx.doi.org/10.1038/embor.2008.7; PMID: 18274550
- Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 2003; 194:237 - 55; http://dx.doi.org/10.1002/jcp.10208; PMID: 12548545
- Fre S, Bardin A, Robine S, Louvard D. Notch signaling in intestinal homeostasis across species: the cases of Drosophila, Zebrafish and the mouse. Exp Cell Res 2011; 317:2740 - 7; http://dx.doi.org/10.1016/j.yexcr.2011.06.012; PMID: 21745469
- Vooijs M, Liu Z, Kopan R. Notch: architect, landscaper, and guardian of the intestine. Gastroenterology 2011; 141:448 - 59; http://dx.doi.org/10.1053/j.gastro.2011.06.003; PMID: 21689653
- Miyamoto S, Rosenberg DW. Role of Notch signaling in colon homeostasis and carcinogenesis. Cancer Sci 2011; 102:1938 - 42; http://dx.doi.org/10.1111/j.1349-7006.2011.02049.x; PMID: 21801279
- Meng RD, Shelton CC, Li Y-M, Qin L-X, Notterman D, Paty PB, et al. γ-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res 2009; 69:573 - 82; http://dx.doi.org/10.1158/0008-5472.CAN-08-2088; PMID: 19147571
- Reedijk M, Odorcic S, Zhang H, Chetty R, Tennert C, Dickson BC, et al. Activation of Notch signaling in human colon adenocarcinoma. Int J Oncol 2008; 33:1223 - 9; PMID: 19020755
- Strosberg JR, Yeatman T, Weber J, Coppola D, Schell MJ, Han G, et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur J Cancer 2012; 48:997 - 1003; http://dx.doi.org/10.1016/j.ejca.2012.02.056; PMID: 22445247
- Arcaroli JJ, Powell RW, Varella-Garcia M, McManus M, Tan AC, Quackenbush KS, et al. ALDH+ tumor-initiating cells exhibiting gain in NOTCH1 gene copy number have enhanced regrowth sensitivity to a γ-secretase inhibitor and irinotecan in colorectal cancer. Mol Oncol 2012; 6:370 - 81; http://dx.doi.org/10.1016/j.molonc.2012.03.004; PMID: 22521243
- Massagué J. TGF-beta signal transduction. Annu Rev Biochem 1998; 67:753 - 91; http://dx.doi.org/10.1146/annurev.biochem.67.1.753; PMID: 9759503
- Schmierer B, Hill CS. TGFB-SMAD signal transduction: molecular specificity and functional flexibility. Mol Cell Biol 2007; 8:970 - 82
- Kodach LL, Wiercinska E, de Miranda NF, Bleuming SA, Musler AR, Peppelenbosch MP, et al. The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Gastroenterology 2008; 134:1332 - 41; http://dx.doi.org/10.1053/j.gastro.2008.02.059; PMID: 18471510
- Grady WM, Myeroff LL, Swinler SE, Rajput A, Thiagalingam S, Lutterbaugh JD, et al. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res 1999; 59:320 - 4; PMID: 9927040
- Miyaki M, Iijima T, Konishi M, Sakai K, Ishii A, Yasuno M, et al. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene 1999; 18:3098 - 103; http://dx.doi.org/10.1038/sj.onc.1202642; PMID: 10340381
- Reinacher-Schick A, Baldus SE, Romdhana B, Landsberg S, Zapatka M, Mönig SP, et al. Loss of Smad4 correlates with loss of the invasion suppressor E-cadherin in advanced colorectal carcinomas. J Pathol 2004; 202:412 - 20; http://dx.doi.org/10.1002/path.1516; PMID: 15095268
- Freeman TJ, Smith JJ, Chen X, Washington MK, Roland JT, Means AL, et al. Smad4-mediated signaling inhibits intestinal neoplasia by inhibiting expression of β-catenin. Gastroenterology 2012; 142:562 - 71, e2; http://dx.doi.org/10.1053/j.gastro.2011.11.026; PMID: 22115830
- Alazzouzi H, Alhopuro P, Salovaara R, Sammalkorpi H, Järvinen H, Mecklin JP, et al. SMAD4 as a prognostic marker in colorectal cancer. Clin Cancer Res 2005; 11:2606 - 11; http://dx.doi.org/10.1158/1078-0432.CCR-04-1458; PMID: 15814640
- Lorente-Trigos A, Varnat F, Melotti A, Ruiz i Altaba A. BMP signaling promotes the growth of primary human colon carcinomas in vivo. J Mol Cell Biol 2010; 2:318 - 32; http://dx.doi.org/10.1093/jmcb/mjq035; PMID: 21098050
- Theunissen J-W, de Sauvage FJ. Paracrine Hedgehog signaling in cancer. Cancer Res 2009; 69:6007 - 10; http://dx.doi.org/10.1158/0008-5472.CAN-09-0756; PMID: 19638582
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature 2001; 411:349 - 54; http://dx.doi.org/10.1038/35077219; PMID: 11357142
- Katoh Y, Katoh M. Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review). [review] Int J Mol Med 2006; 18:1019 - 23; PMID: 17089004
- Weiss GJ, Korn RL. Metastatic basal cell carcinoma in the era of hedgehog signaling pathway inhibitors. [Epub ahead of print] Cancer 2012; http://dx.doi.org/10.1002/cncr.27532; PMID: 22511370
- Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med 2012; 366:2180 - 8; http://dx.doi.org/10.1056/NEJMoa1113538; PMID: 22670904
- Varnat F, Zacchetti G, Ruiz i Altaba A. Hedgehog pathway activity is required for the lethality and intestinal phenotypes of mice with hyperactive Wnt signaling. Mech Dev 2010; 127:73 - 81; http://dx.doi.org/10.1016/j.mod.2009.10.005; PMID: 19861162
- Axelrod JD, Matsuno K, Artavanis-Tsakonas S, Perrimon N. Interaction between Wingless and Notch signaling pathways mediated by dishevelled. Science 1996; 271:1826 - 32; http://dx.doi.org/10.1126/science.271.5257.1826; PMID: 8596950
- Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Martí E. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development 2008; 135:237 - 47; http://dx.doi.org/10.1242/dev.012054; PMID: 18057099
- Watt FM. Unexpected Hedgehog-Wnt interactions in epithelial differentiation. Trends Mol Med 2004; 10:577 - 80; http://dx.doi.org/10.1016/j.molmed.2004.10.008; PMID: 15567325
- van den Brink GR, Bleuming SA, Hardwick JC, Schepman BL, Offerhaus GJ, Keller JJ, et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat Genet 2004; 36:277 - 82; http://dx.doi.org/10.1038/ng1304; PMID: 14770182
- Estrach S, Ambler CA, Lo Celso C, Hozumi K, Watt FM. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development 2006; 133:4427 - 38; http://dx.doi.org/10.1242/dev.02644; PMID: 17035290
- Chen X, Stoeck A, Lee SJ, Shih IeM, Wang MM, Wang T-L. Jagged1 expression regulated by Notch3 and Wnt/β-catenin signaling pathways in ovarian cancer. Oncotarget 2010; 1:210 - 8; PMID: 20953350
- Wall DS, Mears AJ, McNeill B, Mazerolle C, Thurig S, Wang Y, et al. Progenitor cell proliferation in the retina is dependent on Notch-independent Sonic hedgehog/Hes1 activity. J Cell Biol 2009; 184:101 - 12; http://dx.doi.org/10.1083/jcb.200805155; PMID: 19124651
- Sang L, Roberts JM, Coller HA. Hijacking HES1: how tumors co-opt the anti-differentiation strategies of quiescent cells. Trends Mol Med 2010; 16:17 - 26; http://dx.doi.org/10.1016/j.molmed.2009.11.001; PMID: 20022559
- Wall DS, Wallace VA. Hedgehog to Hes1: the heist of a Notch target. Cell Cycle 2009; 8:1301 - 2; http://dx.doi.org/10.4161/cc.8.9.8284; PMID: 19342879
- Tian Q, He X-C, Hood L, Li L. Bridging the BMP and Wnt pathways by PI3 kinase/Akt and 14-3-3ζ. Cell Cycle 2005; 4:215 - 6; http://dx.doi.org/10.4161/cc.4.2.1412; PMID: 15655376
- Fuxe J, Vincent T, Garcia de Herreros A. Transcriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle 2010; 9:2363 - 74; http://dx.doi.org/10.4161/cc.9.12.12050; PMID: 20519943
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005; 435:959 - 63; http://dx.doi.org/10.1038/nature03659; PMID: 15959515
- Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V, et al. Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nat Cell Biol 2011; 13:1244 - 51; http://dx.doi.org/10.1038/ncb2313; PMID: 21841793
- Ruiz i Altaba A. Hedgehog signaling and the Gli code in stem cells, cancer, and metastases. Sci Signal 2011; 4:pt9; http://dx.doi.org/10.1126/scisignal.2002540; PMID: 22114144
- Beck SE, Carethers JM. BMP suppresses PTEN expression via RAS/ERK signaling. Cancer Biol Ther 2007; 6:1313 - 7; PMID: 18059158
- Beck SE, Jung BH, Del Rosario E, Gomez J, Carethers JM. BMP-induced growth suppression in colon cancer cells is mediated by p21WAF1 stabilization and modulated by RAS/ERK. Cell Signal 2007; 19:1465 - 72; http://dx.doi.org/10.1016/j.cellsig.2007.01.017; PMID: 17317101
- Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget 2011; 2:135 - 64; PMID: 21411864
- Jeong WJ, Yoon J, Park JC, Lee SH, Lee SH, Kaduwal S, et al. Ras stabilization through aberrant activation of Wnt/β-catenin signaling promotes intestinal tumorigenesis. Sci Signal 2012; 5:1 - 12; http://dx.doi.org/10.1126/scisignal.2002242; PMID: 22234611
- Pacheco-Pinedo EC, Morrisey EE. Wnt and Kras signaling-dark siblings in lung cancer. Oncotarget 2011; 569-74.
- Mazumdar T, DeVecchio J, Agyeman A, Shi T, Houghton JA. The GLI genes are the molecular switch in disrupting Hedgehog signaling in colon cancer. Oncotarget 2011; 638-45.
- Chen X, Liao J, Lu Y, Duan X, Sun W. Activation of the PI3K/Akt pathway mediates bone morphogenetic protein 2-induced invasion of pancreatic cancer cells Panc-1. Pathol Oncol Res 2011; 17:257 - 61; http://dx.doi.org/10.1007/s12253-010-9307-1; PMID: 20848249
- Hart LS, Dolloff NG, Dicker DT, Koumenis C, Christensen JG, Grimberg A, et al. Human colon cancer stem cells are enriched by insulin-like growth factor-1 and are sensitive to figitumumab. Cell Cycle 2011; 10:2331 - 8; http://dx.doi.org/10.4161/cc.10.14.16418; PMID: 21720213
- Ho L, Alman B. Protecting the hedgerow: p53 and hedgehog pathway interactions. Cell Cycle 2010; 9:506 - 11; http://dx.doi.org/10.4161/cc.9.3.10552; PMID: 20081367
- Efstratiadis A, Szabolcs M, Klinakis A. Notch, Myc and breast cancer. Cell Cycle 2007; 6:418 - 29; http://dx.doi.org/10.4161/cc.6.4.3838; PMID: 17329972
- Julien S, Merino-Trigo A, Lacroix L, Pocard M, Goere D, Mariani P, et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. [Epub ahead of print] Clin Cancer Res 2012; http://dx.doi.org/10.1158/1078-0432.CCR-12-0372; PMID: 22825584
- Xu X, Hou Y, Yin X, Bao L, Tang A, Song L, et al. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell 2012; 148:886 - 95; http://dx.doi.org/10.1016/j.cell.2012.02.025; PMID: 22385958
- Hou Y, Song L, Zhu P, Zhang B, Tao Y, Xu X, et al. Single-cell exome sequencing and monoclonal evolution of a JAK2-negative myeloproliferative neoplasm. Cell 2012; 148:873 - 85; http://dx.doi.org/10.1016/j.cell.2012.02.028; PMID: 22385957
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487:330 - 7; http://dx.doi.org/10.1038/nature11252; PMID: 22810696
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366:883 - 92; http://dx.doi.org/10.1056/NEJMoa1113205; PMID: 22397650