Despite intense investigation on apoptosis pathways, exactly how apoptosis is regulated in various primary cells remains understudied and continues to reveal unexpected mechanisms. While a strict regulation of apoptosis is critical for the long-term survival of postmitotic cells, mitotic cells need to maintain their ability to activate apoptosis rapidly, as they can be at continual risk of becoming cancerous.Citation1 Therefore, cells must efficiently balance the need for having a primed apoptotic pathway vs. the risks associated with inadvertent cell death. This balance is particularly important during embryogenesis, where human embryonic stem (hES) cells proliferate rapidly and differentiate, leading to the development of an entire organism.Citation2 While optimal hES cell survival is necessary for development, the ability of these cells to respond rapidly to DNA damage by apoptosis and maintain genomic integrity is also critical to prevent propagation of mutations in the developing embryo.Citation3 Indeed, hES cells are known to be highly sensitive to DNA damage,Citation4,Citation5 and our recent results have uncovered a novel mechanism by which these cells are primed for rapid apoptosis.Citation6
An essential mediator of apoptosis in mammalian cells is Bax, a proapoptotic member of the Bcl-2 family. In healthy cells, Bax is predominantly cytosolic and present in an inactive conformation. Apoptotic stimuli, such as DNA damage, result in the induction of BH3-only family proteins that promote Bax activation by conformational changes. Activated Bax then translocates to the mitochondria, where it inserts into the mitochondrial outer membrane, promoting the release of cytochrome c and resulting in caspase activation.Citation7 Thus, Bax activation is a critical event in the commitment of cells to apoptosis.
We set out to decipher the mechanism underlying the rapid death response to etoposide-induced DNA damage in hES cells. This death was completely dependent on Bax. Surprisingly, we found Bax to be in its already active state in untreated hES cells.Citation6 These results were unexpected, because, thus far, Bax activation has been seen only in cells that are actively undergoing apoptosis.Citation8 However, we found no evidence of cell death in untreated hES cells that have activated Bax. Various elegant structural studies have shown that binding of the BH3-only activators to Bax induces conformational changes in Bax that expose the N terminus and the BH3 domain and mobilize the C terminus to insert into the mitochondrial outer membrane.Citation9 Our results show that these steps of Bax activation are uncoupled in hES cells, with the N terminus of Bax already exposed and stabilized but without triggering of downstream structural events.
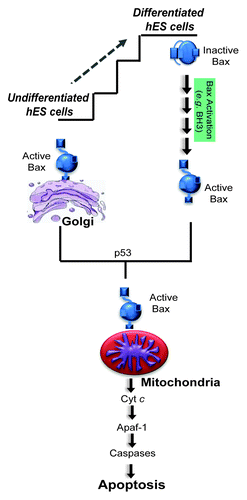
In cells undergoing apoptosis, active Bax localizes to the mitochondria to induce caspase activation.Citation7 Unexpectedly, we found active Bax to be localized not at the mitochondria but at the Golgi (specifically at the trans-Golgi network) in untreated hES cells.Citation6 These results identify hES cells as the first primary cell type to maintain Bax in its active state in the absence of cell death. Importantly, by localizing active Bax at the Golgi, hES cells appear to have developed a mechanism that allows them to maintain Bax in its active state but avoid the risk of spontaneous apoptosis associated with its mitochondrial localization.
To determine whether the constitutively active Bax localized to the Golgi enables hES cells to undergo rapid apoptosis, we examined whether active Bax changes localization after DNA damage. As early as 3 h after etoposide treatment, active Bax translocated from the Golgi to the mitochondria in hES cells undergoing apoptosis. Importantly, active Bax was maintained at the Golgi after etoposide treatment in p53-knockdown hES cells, indicating that p53 was required for the Golgi-to-mitochondria translocation of active Bax after DNA damage.Citation6 Interestingly, high-resolution microscopy shows that the mitochondrial and Golgi networks in hES cells are very closely associated (almost intertwined), and this spatial proximity likely facilitates the rapid translocation of Bax from Golgi to mitochondria (unpublished results).
Finally, as hES cells have the potential to be differentiated into all cell lineages,Citation10 we examined whether the constitutively active status of Bax seen in undifferentiated hES cells changed with differentiation. Interestingly, Bax was no longer present in its active state after just two days of differentiation. Consistent with the idea that the presence of active Bax in undifferentiated hES cells primes them for rapid apoptosis, the 2-day differentiated hES cells were also no longer acutely sensitive to DNA damage.Citation6 These results illustrate how the apoptotic machinery undergoes dynamic changes to set apoptotic thresholds even at the earliest stages of hES cell differentiation ().
These findings provide insight into how hES cells are primed to undergo rapid apoptosis, thus avoiding the accumulation of genomic mutations during a critical period of proliferation in early development. Many intriguing questions remain: what is the specific mechanism by which Bax is maintained in an active state in undifferentiated hES cells? How does active Bax localize to the Golgi? What is the molecular trigger that mobilizes active Bax from the Golgi to the mitochondria? How does the differentiation program reset the apoptotic sensitivity of hES cells? These investigations will undoubtedly uncover critical aspects of apoptosis regulation in cells and reveal key features of stem cell biology that may have significant impact for regenerative medicine.
Figure 1. Undifferentiated hES cells have a primed death machinery with Bax already active and localized to the Golgi. In response to DNA damage, undifferentiated hES cells die by 5 h. DNA damage induces the rapid translocation of active Bax from Golgi to mitochondria in a p53-dependent manner. Differentiation of hES cells resets the apoptotic program where Bax is no longer active and the cells are no longer highly sensitive to DNA damage. Once Bax translocates to the mitochondria, the following steps of apoptosis seem to proceed in a similar manner in both undifferentiated and differentiated cells.
References
- Wright KM, et al. Cell Cycle 2006; 5:1616 - 20; http://dx.doi.org/10.4161/cc.5.15.3129; PMID: 16880745
- Thomson JA, et al. Science 1998; 282:1145 - 7; http://dx.doi.org/10.1126/science.282.5391.1145; PMID: 9804556
- Stambrook PJ, et al. Preservation of Genomic Integrity in Mouse Embryonic Stem Cells, The Cell Biology of Stem Cells. In: Meshorer E, Plath K, eds.: Springer US, 2010:59-75.
- Momcilović O, et al. Stem Cells 2009; 27:1822 - 35; http://dx.doi.org/10.1002/stem.123; PMID: 19544417
- Grandela C, et al. Stem Cell Res 2007; 1:116 - 28; http://dx.doi.org/10.1016/j.scr.2007.10.003; PMID: 19383392
- Dumitru R, et al. Mol Cell 2012; 46:573 - 83; http://dx.doi.org/10.1016/j.molcel.2012.04.002; PMID: 22560721
- Tait SW, et al. Nat Rev Mol Cell Biol 2010; 11:621 - 32; http://dx.doi.org/10.1038/nrm2952; PMID: 20683470
- Youle RJ, et al. Nat Rev Mol Cell Biol 2008; 9:47 - 59; http://dx.doi.org/10.1038/nrm2308; PMID: 18097445
- Gavathiotis E, et al. Cell Cycle 2011; 10:868 - 70; http://dx.doi.org/10.4161/cc.10.6.15034; PMID: 21325897
- Odorico JS, et al. Stem Cells 2001; 19:193 - 204; http://dx.doi.org/10.1634/stemcells.19-3-193; PMID: 11359944