Abstract
Nek9 (also known as Nercc1), a member of the NIMA (never in mitosis A) family of protein kinases, regulates spindle formation, chromosome alignment and segregation in mitosis. Here, we showed that Nek9 protein was expressed from germinal vesicle (GV) to metaphase II (MII) stages in mouse oocytes with no detectable changes. Confocal microscopy identified that Nek9 was localized to the spindle poles at the metaphase stages and associated with the midbody at anaphase or telophase stage in both meiotic oocytes and the first mitotic embyros. Depletion of Nek9 by specific morpholino injection resulted in severely defective spindles and misaligned chromosomes with significant pro-MI/MI arrest and failure of first polar body (PB1) extrusion. Knockdown of Nek9 also impaired the spindle-pole localization of γ-tubulin and resulted in retention of the spindle assembly checkpoint protein Bub3 at the kinetochores even after 10 h of culture. Live-cell imaging analysis also confirmed that knockdown of Nek9 resulted in oocyte arrest at the pro-MI/MI stage with abnormal spindles, misaligned chromosomes and failed polar body emission. Taken together, our results suggest that Nek9 may act as a MTOC-associated protein regulating microtubule nucleation, spindle organization and, thus, cell cycle progression during mouse oocyte meiotic maturation, fertilization and early embryo cleavage.
Introduction
Mouse oocyte maturation is a multi-stage, precisely orchestrated and orderly process.Citation1,Citation2 Germinal vesicle breakdown (GVBD) marks the beginning of oocyte maturation. After GVBD, microtubules assemble around chromosomes during the pro-metaphase I stage, and then chromosomes migrate to the central plate of the bipolar spindle at the MI stage. Subsequently, the spindle migrates to the cortex and the oocyte emits the first polar body, followed by the formation of the metaphase II (MII) spindle located beneath the plasma membrane.Citation3 The unfertilized mouse oocyte contains over 80 small microtubule-organizing centers (MTOCs) within the cytoplasm. In mitosis, the centrosomes form the two poles of the mitotic spindle and contribute to nucleation and organization of the highly dynamic spindle microtubules (MTs).Citation4 MTOCs vary in shape and size, but all contain the pericentriolar material (PCM) components: γ-tubulin and pericentrin, a critical protein that anchors γ-tubulin at the PCM in somatic cells. At prometaphase, MTOCs begin to accumulate to form spindle poles and then locate at both poles in a distinct “O” or “C” shape at the metaphase.Citation5,Citation6 While oocyte MTOC function is similar to that of centrosomes, and MTOCs are essential for meiotic spindle assembly, the underlying mechanisms that regulate the recruitment of pericentriolar material proteins for the biogenesis and organization of MTOCs and the regulation of microtubule nucleation and assembly are not well understood.Citation7,Citation8 Recent studies indicate that besides the role of cyclin-dependent kinases (CDK) in cell division, several other families of protein kinases, including Polo-like kinases (PLK), Aurora kinases and NIMA-like kinases (Neks) also play important roles at different stages of this intricate process.Citation9-Citation14 NIMA-like kinases are named for the Aspergillus nidulans protein kinase encoded by the NIMA (never in mitosis Aspergillus) gene that participates in a broad array of mitotic processes.Citation15,Citation16 The mammalian genome encodes 11 protein kinases, the catalytic domain of which is evolutionarily related to that of NIMA, but each diverges substantially from NIMA in its non-catalytic C-terminal tail.Citation16,Citation17 In Aspergillus nidulans it has been demonstrated that NIMA is required for cell cycle progression into mitosis. Cyclin B plays a role in the mitosis-specific activation of NIMA, and both cyclin B and NIMA have to be properly activated before mitosis can be initiated in this species.Citation15,Citation18 Available reports also indicate that the NIMA-family kinases are the essential molecules in mitosis progression and are involved in many processes, such as centrosome separation, chromosome condensation in prophase, nuclear envelope breakdown and spindle assembly in pro-metaphase, as well as in exit from mitosis and cytokinesis.Citation19-Citation21 Nek9 is a 107-kDa polypeptide whose N-terminal catalytic domain is followed by a domain homologous to RCC1, the exchange factor for the small G protein Ran. The RCC1 domain of Nercc1 acts as an auto-inhibitory domain through the direct binding to Nek9 protein kinase domain during interphase.Citation22 Mammalian Nek9 coimmunoprecipitates with γ-tubulin, and the activated Nek9 polypeptides localize to the centrosomes and spindle poles during early cell division, suggesting that active Nek9 has important functions at the microtubule organizing center during mitosis.Citation23 Nek6 and Nek7 can bind strongly to the C-terminal tail of Nek9 distal to the RCC domain.Citation22 Moreover, Nek6, which is itself a mitotic kinase, can be directly phosphorylated and activated by Nek9 in vivo and in vitro.Citation24 Both Nek6 and Nek7 kinases activation contributes to mitotic progression downstream of Nek9, and interfering with their activity by either knockdown or expression of reduced-activity mutants leads to mitotic arrest and apoptosis.Citation25 Nek9 activation by PLK1 contributes to the phosphorylation of the mitotic kinesin Eg5 at Ser1033, which, together with the CDK1, is necessary for subsequent centrosome separation and timely mitosis.Citation26 Although Nek9 plays key roles in mitosis, whether Nek9 participates in acentriolar meiotic spindle assembly and subsequent accurate chromosome segregation remains unknown. In the present study, we for the first time investigated the localization and functions of Nek9 during mouse oocyte meiotic maturation and early embryo cleavage. Our results reveal that Nek9 might act as a centrosome-associated protein to play a crucial role in spindle assembly, spindle pole formation, chromosome alignment and the first polar body extrusion during mouse oocyte meiotic maturation. The depletion of Nek9 decreased recruitment of γ-tubulin to MTOCs and activated the spindle checkpoint, resulting in the arrest of oocyte maturation at the Pro-MI/MI stage.
Results
Expression and subcellular localization of Nek9 during mouse oocyte meiotic maturation
Oocytes were cultured for 0, 4, 8 and 12 h, corresponding to germinal vesicle (GV), pro-metaphase I (pro-MI), metaphase I (MI) and MII stages, respectively. Immunoblotting results showed that Nek9 protein was expressed from GV to MII stages, without detectable changes (). The molecular mass of Nek9 is about 107 kDa, and that of β-actin is 43 kDa. To investigate the subcellular-specific localization of Nek9 during meiotic maturation, mouse oocytes at different stages of maturation were cultured and processed for immunofluorescent staining. As shown in , Nek9 was mainly distributed in the germinal vesicle at the GV stage. After GV breakdown (GVBD, 2 h of culture), Nek9 began to accumulate in the vicinity of condensed chromosomes. At the pro-metaphase I stage, Nek9 gradually migrated to the spindle poles before the condensed chromosomes were aligned at the equator of the spindle. When oocytes progressed to metaphase I and the chromosomes were aligned at the equatorial plate, Nek9 was stably presented at the spindle poles. At the stage of anaphase I/telophase I, Nek9 was stained in the region between the separating homologous chromosomes, and associated with the midbody between the first polar body and the oocyte. At the MII stage, Nek9 was again localized at the spindle poles ().
Figure 1. Expression and subcellular localization of Nek9 during mouse oocyte meiotic maturation. (A) Expression of Nek9 was detected by western blotting. Samples were collected after culture for 0, 4, 8 and 12 h, the time points when most oocytes reached the GV, PRO1, MI and MII stages, respectively. The molecular mass of Nek9 and β-actin were about 107 and 43 kDa, respectively. Each sample was collected from 250 oocytes. (B) Subcellular localization of Nek9 as shown by immunofluorescent staining. Oocytes at various stages were stained with an antibody against Nek9 (green); each sample was counterstained with PI to visualize DNA (red). GV, oocytes at germinal vesicle stage; GVBD, oocytes at germinal vesicle breakdown; ProI, oocytes at first prometaphase; MI, oocytes at first metaphase; AnaI, oocytes at first anaphase; TelI, oocytes at first telophase; MII, oocytes at second metaphase. An MII oocyte was used as a negative control for Nek9 confocal microscopy, in which no first antibody was used but the fluorescent second antibody was used. Bar = 20 µm (C) Colocalization of γ-tubulin and Nek9 in GVBD, ProI, MI and MII stages. Oocytes cultured for 8 h (MI) and 12 h (MII) were fixed and stained for γ-tubulin (pink), Nek9 (green) and DNA (red). Bar = 20 µm.
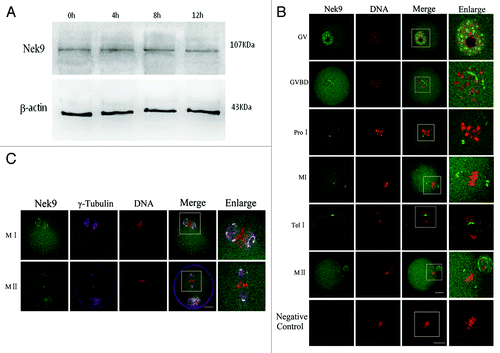
The apparent association of Nek9 with spindle poles prompted us to ask whether Nek9 was co-localized with other well-known MTOC-associated proteins, such as γ-tubulin. As shown in , the signals of Nek9 completely overlapped with those of γ-tubulin during the entire meiotic maturation from GVBD to MII stages. Co-localization of Nek9 with γ-tubulin further confirmed that the localization of Nek9 was at the two spindle poles ().
Subcellular localization of Nek9 during fertilization and early embryo cleavage
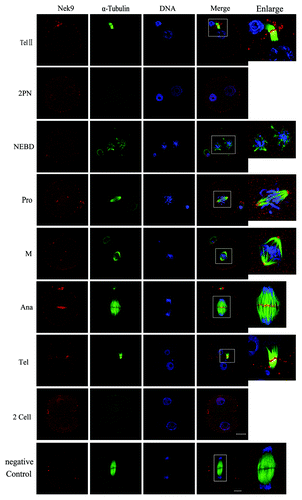
After insemination, the oocytes entered telophase II and extruded their second polar body. The chromatids separated, and Nek9 moved to the midbody (, TelII). In the fertilized eggs, many Nek9 dots were distributed within the pronuclei. After nuclear envelope breakdown (NEBD), Nek9 began to form around the periphery of chromosomes until the MI spindle was formed. At prophase and M phase, Nek9 was stably stained at the first mitotic spindle poles (, Pro and M). When the zygote progressed into Ana and Tel stages, Nek9 moved to the middle area between the separated chromatids (, Ana) or midbody (, Tel) just like in TelI and TelII in meiosis. By the completion of the first mitotic cell cycle, two blastomeres formed, and both of them entered interphase. At this stage, again Nek9 signals were stained in the nucleus, similar to the stage of 2PN ().
Figure 2. Subcellular localization of Nek9 during fertilization and early embryo cleavage. Subcellular localization of Nek9 during fertilization and early embryo cleavage by immunofluorescent staining. Oocytes at various stages were stained with an antibody against Nek9 (red), α-tubulin (green) and DNA (blue). TelII, zygote at telophaseII stage; NEBD, zygote with one nuclear envelope breakdown (NEBD); Pro, zygote at the stage of pro-metaphase; M, zygote at the stage of metaphase; Ana, zygote at the stage of anaphase; Tel, zygote at the stage of telophase; two-cell embryo. A zygote at the anaphase stage was used as a negative control for confocal microscopy, in which no first antibody was used but the fluorescent second antibody was used. Bar = 20 µm.
Subcellular localization of Nek9 in mouse oocytes treated with taxol or nocodazole
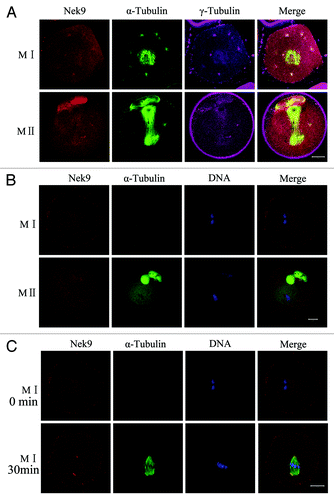
Oocytes treated with taxol or nocodazole were triple-stained for Nek9, γ-tubulin and α-tubulin. As presented in , the microtubule fibers in taxol-treated oocytes were excessively polymerized, leading to significantly enlarged spindles, together with many asters in the cytoplasm. In taxol-treated experiments, both Nek9 and γ-tubulin signals overlapped at the abnormal spindle poles as well as in the cytoplasmic asters. In nocodazole-treated oocytes, microtubules were completely disassembled, and no intact spindles could be observed. The localization of Nek9 was altered and dispersed into the cytoplasm (). When the nocodazole-treated oocytes at the MI stage were thoroughly washed and cultured for 30 min to allow microtubule re-assembly, Nek9 appeared again at the poles of the reformed spindle apparatus ().
Figure 3. Localization of Nek9 in mouse oocytes treated with spindle perturbing agents during meiotic maturation. (A) Oocytes at the MI and MII stages were incubated in M2 medium containing 10 μM taxol for 45 min and then triple-stained with antibodies against Nek9, γ-tubulin and anti-α-tubulin-FITC. Both Nek9 and γ-tubulin signals were localized at the cytoplasmic asters and spindle poles of the same oocytes. α-tubulin, green; Nek9, red; γ-tubulin, pink. Bar = 20 μm. (B) Localization of Nek9 and α-tubulin in oocytes treated with nocodazole. Oocytes at the MI and MII stage were incubated in M2 medium containing 20 μg/ml nocodazole for 10 min and then stained for Nek9 (red), α-tubulin (green) and DNA (blue). Bar = 20 μm. (C) Oocytes at the MI stage, first treated with nocodazole, then washed thoroughly and recovered for 30 min. Finally, oocytes were fixed and stained for α-tubulin (green), Nek9 (red) and DNA (blue). Bar = 20 μm.
Depletion of Nek9 causes abnormal spindle and misaligned chromosomes
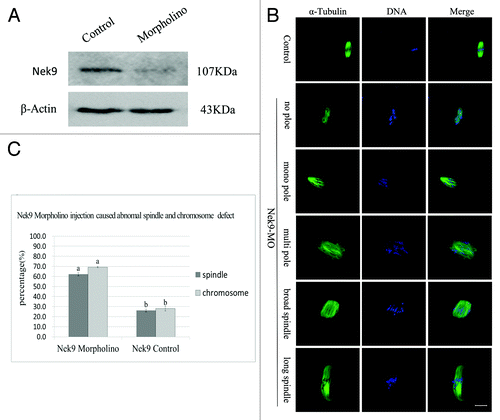
To further disclose the roles of Nek9 in mouse oocyte meiotic maturation, we knocked down Nek9 by its specific morpholino (MO) injection. Western blotting analysis showed that the expression level of Nek9 was notably reduced (), which revealed the efficiency of the Nek9 depletion. In the Nek9-MO injection group, the oocytes exhibited different kinds of morphologically defective spindles and misaligned chromosomes. The major spindle defects were abnormal spindle poles (52%, n = 94), including spindles with no pole, one pole, multi-poles and others with elongated and malformed spindles with astral microtubules (). Chromosome misalignment was also observed (). As shown in , the rate of abnormal spindles in the Nek9-MO injection group (62.2 ± 1.6%, n = 94) was significantly higher than that in the control-MO injection group (26.1 ± 1.3%, n = 92) (p < 0.05). Furthermore, the rate of misaligned chromosomes in Nek9 MO (69.2 ± 0.6%, n = 94) and control groups (28.1 ± 2.3%, n = 92) were significantly different (p < 0.05, ).
Figure 4. Depletion of Nek9 causes abnormal spindle and misaligned chromosomes in MI oocytes. After microinjection of Nek9 or control MO, the oocytes were incubated in M2 medium containing 2.5 µM milrinone for 24 h, then washed thoroughly and transferred to milrinone-free M2 medium for 8.5 h, followed by western blotting. (A) Western blot of Nek9 in the Nek9 MO group and control group. The molecular mass of Nek9 is 107 kDa and that of β-actin is 43 kDa. (B) Oocytes microinjected with Nek9 or control MO were incubated in M2 medium containing 2.5 µM milrinone for 24 h, and then transferred to milrinone-free M2 for 8.5 h, followed by immunostaining with α-tubulin antibody (green) and Hoechst (blue). In the Nek9 MO injection group, the oocytes exhibited various morphologically defective spindles and misaligned chromosomes. The major defects showed abnormal spindle poles including spindles without pole, one pole, multi-poles and malformed spindles with astral microtubules as well as many cytoplasmic asters. Bar = 20 μm. (C) The rate of oocytes with abnormal spindles or misaligned chromosomes in the Nek9 MO injection group and control group. Data are presented as means ± SEM of three independent experiments. Different letters indicate statistical difference (p < 0.05).
Knockdown of Nek9 causes dissociation of γ-tubulin from spindle poles
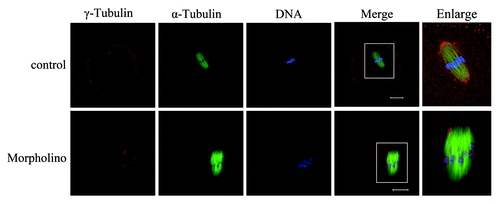
The above results revealed that Nek9 might be involved in the spindle organization and spindle pole formation. As is well-known, the MTOC-associated protein γ-tubulin is a key regulator in microtubule organization and spindle formation during mouse oocyte meiosis.Citation27,Citation28 Since Nek9 co-localized with γ-tubulin during meiotic maturation, we further investigated the effect of Nek9 depletion on γ-tubulin localization. Strikingly, by employing MO injection, we found that Nek9 depletion markedly affected the localization of γ-tubulin. As shown in , γ-tubulin was localized to the spindle poles in control-MO-injected oocytes at the MI stage, while in Nek9-MO-injected oocytes, γ-tubulin was no longer accumulated at the spindle poles, being irregularly dispersed to the spindle fibers or distributed into the cytoplasm.
Figure 5. Knockdown of Nek9 causes dissociation of γ-tubulin from spindle poles. Oocytes injected with Nek9 MO or control MO were cultured in fresh M2 medium for 8 h, followed by double-staining of α-tubulin (green), γ-tubulin (red) and DNA (blue). In the control MO-injected group, γ-tubulin was associated with the spindle poles at the metaphase I stage, whereas in the Nek9 MO-injected group, γ-tubulin dissociated from abnormal spindle poles and dispersed into the cytoplasm. α-tubulin, green; γ-tubulin, red; DNA, blue. Bar = 20 μm.
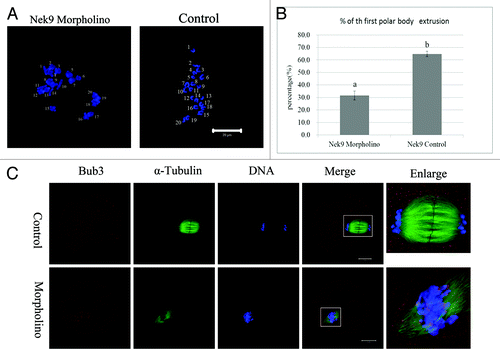
Nek9 depletion causes Pro-MI/MI arrest, reduced PB1 extrusion and activation of the SAC protein
After Nek9 or control MO injection, oocytes were placed in M2 medium containing 2.5 µM milrinone for 24 h. Then, oocytes were continuously cultured in milrinone-free M2 medium for 12 h. Analysis of the first polar body (PB1) emission using confocal microscopy revealed that most oocytes injected with control morpholino extruded the first polar body and reached the MII stage containing normal-shaped spindles with well-aligned chromosomes. In sharp contrast, the majority of Nek9-depleted oocytes were arrested at the Pro-MI/MI stage, and few oocytes extruded the first polar body by 12 h. As shown in , the PB1 extrusion rate in the Nek9 morpholino-injected group (31.6 ± 3.6%, n = 113) was considerably lower than that in the control-MO group (64.8 ± 2.2%, n = 132, p < 0.05). Since Nek9 depletion disrupted the spindle assembly, with the majority of oocytes blocked in Pro-MI/MI arrest, we asked whether the chromosomes could undergo correct segregation. Oocytes in both Nek9 MO and control groups were cultured for 12 h. Chromosome-spreading experiments confirmed the failed chromosome separation. Chromosome spreading showed that chromosomes were still in the bivalent state in Nek9-depleted oocytes (10/10); in contrast, univalent chromosomes could be seen in control oocytes (9/9), indicating completion of anaphase (). Next, we analyzed the localization of Bub3 in oocytes from the Nek9-MO group to explore the activation of SAC proteins. Specific signals for Bub3 were detected in the Pro-MI/MI-arrested oocytes in the Nek9-MO group even after 10 h culture. In contrast, the control oocytes entered anaphase and showed no signals of Bub3. Detection of Bub3 signal indicates activation of the spindle assembly checkpoint ().
Figure 6. Nek9 depletion causes Pro-MI/MI arrest and failed PB1 extrusion and activation of SAC. (A) Oocytes of control MO and Nek9 MO groups were cultured for 12 h, followed by chromosome spreading experiments. (B) Percentages of the first polar body extrusion in the Nek9 MO injection group and control MO group. Data are presented as means +SEM of three independent experiments. Different superscripts indicate statistical difference at a p < 0.05 level of significance. (C) Detection of Bub3 in oocytes in Nek9 MO groups. Bub3, red; DNA, blue. Bar = 20 µm.
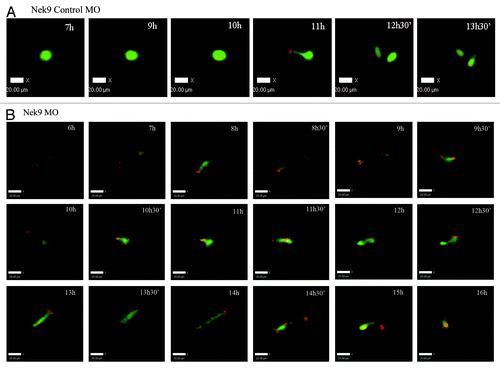
Knockdown of Nek9 prevents chromosome segregation and perturbs the metaphase-anaphase transition as reveled by time-lapse imaging
Live-cell imaging showed that in the control group, the meiotic spindle was visible about 4 h of GVBD and slowly migrated toward the oocyte cortex followed by rapid polar bodyCitation1 extrusion at about 9 h of culture following GVBD (; Fig. S1). In contrast, in the Nek9-MO injection group, various morphologically defective spindles were seen, and chromosomes failed to separate and remained at the Pro-MI/MI stage even at 12 h of GVBD. Additionally, no first polar body extrusion was observed in the Nek9-MO injection group (; Fig. S2).
Figure 7. Nek9 knockdown disturbs the metaphase-anaphase transition of mouse oocytes as revealed by time-lapse live-cell imaging. (A) Oocytes co-injected with β5-tubulin-GFPmRNA and control-MO. Spindle (fluorescent tubulin) and DNA (red) images in a typical control oocyte during in vitro maturation. Time points indicate the time-lapse from GVBD. (B) Similar to (A), oocytes were co-injected with β5-tubulin-GFP mRNA and Nek9-MO. Shown are images of oocytes with abnormal spindles, aberrant chromosomes, futile and unsuccessful chromatin segregation and failure of PB1 extrusion. Tubulin, green; DNA, red.
Discussion
Nek9 was identified as a new NIMA-like kinase that regulates chromosome alignment and segregation in mitosis. Microinjection of anti-Nek9 antibodies in prophase interferes with mitotic spindle organization and inhibits the correct segregation of chromosomes, resulting in either prometaphase arrest or aneuploidy.Citation22 In the present study, we investigated the localization pattern and potential function of Nek9 during mouse oocyte meiotic maturation and early embryo cleavage. We demonstrated that Nek9 is a component of the acentriolar MTOCs in mouse oocytes, which is critical for meiotic spindle stability and accurate chromosome segregation. Depletion of Nek9 in oocytes using specific MO dissociated γ-tubulin from the MTOCs with disrupted meiotic spindle structure and misaligned chromosomes. In turn, this causes sustained spindle assembly checkpoint activation and meiotic arrest at the Pro-MI/MI stage.
It has been shown that Nek9 is localized at the centrosome or mitotic spindle pole, and its activation increases in mitosis.Citation23 In our research, we showed for the first time that Nek9 was spatially correlated with the spindle pole organization and midbody formation during mouse oocyte meiotic maturation, which correlated well to that in mitosis. The location of Nek9 in mammalian meiosis and first embryo cleavage strongly suggests that Nek9 may act as a component of mammalian oocyte MTOCs and play a role in microtubule organization and the meiotic process, very similar to several other MTOC-associated proteins, such as MEK1/2, p38α MAPK, GM130 and NuMA, that we previously demonstrated to be needed for proper spindle assembly in meiosis.Citation29,Citation30,Citation31,Citation32
To study the correlation between Nek9 and microtubule organization, taxol and nocodazole were employed. When mouse oocytes were treated with taxol to promote non-spindle microtubule asters in the cytoplasm, Nek9 foci were detected in the center of these cytoplasmic asters, in addition to those at the spindle poles. In addition, when oocytes were exposed to nocodazole to completely disassemble microtubule, the Nek9 foci dispersed into the cytoplasm. When the treated oocytes were thoroughly washed and cultured to allow microtubule reassembly, the Nek9 signals again appeared at the poles of the reformed spindle apparatus. These results further indicate the involvement of Nek9 in microtubule organization.
Polo-like kinase1 (Plk1) has been shown to be required for regulating multiple stages of both mitotic and meiotic progression, including entry into and exit from the M phase, spindle organization and cytokinesis.Citation33,Citation34 There was evidence that Nek9 acts as a transducer downstream of Plk1. Plk1, alone or in combination with CDK1 (cyclin-dependent kinase1), could phosphorylate Nek9. Nek6, Nek7 and Nek9 act together in a mitotic kinase cascade with Nek9 being upstream of Nek6 and Nek7. Nek9 was identified as an interacting partner of Nek6 and subsequently shown to phosphorylate Nek6 at S206 within its activation loop.Citation26 Nek6 is constitutively associated with Eg5 (also known as Kinesin-5 and Kif11), which is necessary for spindle bipolarity.Citation7 Nek6 phosphorylated Eg5 at several sites in vitro, one of which, Ser1033, can be phosphorylated in vivo during mitosis. Eg5 depletion caused mitotic arrest, resulting in cells with a monopolar spindle.Citation17,Citation35 Interference with Nek6 has been reported by one group to lead to metaphase arrest and apoptosis, which suggested that Nek6 kinase is required for the metaphase-anaphase transition.Citation36 In the present study, Nek9 function in mouse oocyte meiosis was tested using specific MO to knockdown its translation. Depletion of Nek9 protein caused evident disruptions in the meiotic spindle as well as extensive chromosome misalignment.
The detailed mechanisms by which Nek9 functions in spindle assembly remain far from understood. Microtubules are primarily nucleated by centrosomes or MTOCs that are localized at the spindle poles.Citation37 γ-tubulin is a major component of the pericentriolar material of MTOCs. During early mitosis, mammalian Nek9 co-immunoprecipitates with γ-tubulin, and the activated Nek9 protein localizes to the centrosomes and spindle poles.Citation23 Immunodepletion of Nek9 in prophase results in delayed spindle assembly, fewer bipolar spindles, spindle abnormalities and/or chromosomal misalignment.Citation22 NEDD1 (neural precursor cell expressed developmentally downregulated protein1) is another essential protein of acentriolar oocyte MTOCs, which functions in the regulation of meiotic spindle stability.Citation38 The recruitment of γ-tubulin to the spindle pole depends on the adaptor protein NEDD1, and is also controlled by the kinase Plk1.Citation39,Citation40 Nek9 can phosphorylate NEDD1 on Ser377, driving its recruitment and thereby γ-tubulin to the centrosome in mitotic cells.Citation41 It could be inferred that Nek9, through contributing to the recruitment of γ-tubulin, is involved in meiotic spindle assembly.
Microtubule nucleation also occurs in the vicinity of the chromosomes and within the spindle itself. Ran directs the assembly of the spindle, nuclear-envelope dynamics and the mitotic progress.Citation42,Citation43 In mouse oocytes, Ran GTP gradient is necessary for timely meiosis I (MI) and spindle assembly.Citation44 It has been established that Nek9 has a domain homologous to RCC1, a nucleotide exchange factor protein for the Ran GTPase, and also binds specifically to the Ran GTPase.Citation22 Depletion of Nek9 also slows aster assembly induced by Ran-GTP, producing aberrant morphology of Ran-asters.Citation23 In the present study, our data also showed that Nek9 was co-localized with γ-tubulin at MTOCs during the meiotic process. Moreover, depletion of Nek9 dissembled the localization of γ-tubulin from the spindle poles. These data strongly imply that Nek9 may contribute to both the centrosomal and the chromatin/Ran pathways that collaborate in the assembly of a bipolar spindle during meiotic maturation.
During mammalian mitosis and meiosis, SAC is a proofreading network to prevent anaphase onset until all kinetochores of chromosomes are properly attached to the spindle. SAC proteins such as mitotic arrest-deficient-1 (Mad1), Mad2, budding uninhibited by benzimidazole-1 (Bub1), Bub3, BubR1 and monopolar spindle 1 (Mps1) have been shown to play important roles in oocyte meiotic maturation.Citation45-Citation49 Nek9 depletion caused significant chromosome misalignment, abnormal spindle and decreased PBE rates. In addition, chromosome spreads showed that chromosomes were still in the bivalent state in Nek9-MO oocytes arrested at Pro-MI/MI. All these results prompted us to explore whether Nek9 depletion may affect SAC. Specific signals for Bub3 were detected in MI-arrested oocytes in the Nek9 MO group after 10 h culture, while the control oocytes entered the anaphase. Detection of Bub3 indicates unsuccessful attachment of chromosomes to the bipolar spindle with the proper tension.
The relocalization of active Nek9 to the midbody at TelI, TelII in meiosis and at anaphase during early mitosis raises the possibility that Nek9 may have additional functions after the metaphase-to-anaphase transition. The centrosome also contributes significantly to the completion of cytokinesis, through its transport of important components to the midbody. Accumulating data show that many proteins located at the centrosome migrate to the midzone and are likely to participate in cytokinesis. Prolyl isomerase cyclophilin A (cypA) is a centrosome protein that undergoes cell cycle-dependent relocation to the midzone and midbody during cytokinesis. Depletion of cypA leads to cytokinesis defects, and expression of wild-type cypA can reverse the cytokinesis defect.Citation50 AMP-activated protein kinase (AMPK) directly associates with the mitotic apparatus and travels from centrosomes to the spindle midzone during mitosis and cytokinesis.Citation51 Evidence is mounting that Plk1 regulates activation of AMPK and plays roles in cytokinesis.Citation52,Citation53 In addition, Plk1 can phosphorylate Ataxin-10, which is located at the midbody and required for cytokinesis.Citation54 In our study, the relocalization of active Nek9 to the midbody at TelI and TelII indicates that Nek9 is also a core midzone component in mouse oocytes. Nek9-depleted oocytes were arrested at the Pro-MI/MI stage, and few oocytes extruded the first polar body by 12 h. In sharp contrast, most oocytes injected with control morpholino extruded the first polar body and reached the MII stage. These results were further supported by time-lapse microscopy. Whereas oocytes injected with control-MO entered anaphase and extruded the first polar body by 12 h, the Nek9-MO oocytes remained arrested in metaphase for many hours without first polar body extrusion. The failure of PB1 extrusion in Nek9-depleted oocytes indicates a function for Nek9 in cytokinesis.
In conclusion, all our data demonstrate that Nek9 may act as an MTOC-associated protein and cooperates with γ-tubulin to regulate microtubule assembly and spindle organization, which is required for the metaphase-anaphase transition to promote the mouse oocyte meiotic maturation process.
Materials and Methods
Antibodies and reagents
Rabbit polyclonal anti-Nek9 antibody used in western blot and immunofluorescence was purchased from Bioworld company (Cat# BS1845); mouse monoclonal anti-α-tubulin-FITC antibody and mouse monoclonal anti-γ-tubulin antibody were obtained from Sigma-Aldrich Co. (Cat# F2168, Cat# T6557); rabbit polyclonal anti-bub3 antibody was purchased from Santa Cruz Biotechnology (Cat#Sc-28258). FITC- or TRITC-conjugated goat anti-rabbit IgG(H+L) and TRITC-conjugated goat anti-mouse IgG(H+L) were produced by Jackson ImmunoResearch Laboratories, Inc. and subpackaged by Zhongshan Golden Bridge Biotechnology Co. LTD (Cat#ZF-0311,Cat#Zf-0316, Cat#Zf-0313). All other reagents were purchased from Sigma Aldrich except for those specifically mentioned.
Ethics statement
Six- to eight-week-old ICR mice care and use were conducted in accordance with the Animal Research Committee guidelines of the Institute of Zoology (IOZ), CAS. Mice were housed in a temperature-constant room with fixed darkness-light cycles, fed with a regular diet and maintained under the care of the Laboratory Animal Unit, Institute of Zoology, CAS. All animal handling staffs were trained before using animals. The mice were sacrificed by cervical dislocation, and the only procedure performed on the sacrificed mice was the collection of oocytes from the ovaries.
Mouse oocyte collection and culture
Immature oocytes were collected from ovaries of 6–8-wk-old female ICR mice in M2 medium (Sigma) supplemented with or without 2.5 µM milrinone. Milrinone was used to keep oocytes at the GV stage. Only those immature oocytes displaying a GV were further cultured in M2 medium under liquid paraffin oil at 37°C in an atmosphere of 5% CO2 in air. After specific time periods of culture, oocytes were collected for drug treatment, microinjection, western blotting analysis or immunofluorescence.
Zygotes and two-cell embryos collection and culture
ICR females were induced to super-ovulate by intra-peritoneal injection of pregnant mare serum gonadotropin (10 IU, PMSG) at noon, 48 h prior to human chorionic gonadotropin injection (10 IU, hCG) at noon on the day of breeding. ICR stud males were introduced to the female mice at 5 p.m. Females were selected for retrieval of zygotes between 8 and 9 a.m. on the day of the observation of the copulatory plug, i.e., ~8 h post-coitus (pc). To collect zygotes, cumulus-oocyte complexes (COCs) were collected from the ampullar region of oviducts 8 h post-coitus (pc). After cumulus cells were dispersed by adding 1 mg/mL of hyaluronidase for 1 min and removed by gentle pipetting, the inseminated eggs were washed and cultured in 30 µl droplets of potassium simplex optimized medium (KSOM) under liquid paraffin oil at 37°C in humidified air containing 5% CO2. The formation of pronuclei was examined at 12 h of pc, and only those oocytes that extruded second polar bodies and formed pronuclei were used for further study. Starting from 15–16 h of pc, zygotes were checked every 20 min to determine the time for nuclear envelope breakdown (NEBD), and then collected for further research at different periods of NEBD. Embryos undergoing NEBD were defined as 0 min, and were further cultured in KSOM for specific periods to NEBD (0 min), pro-metaphase (60 min), metaphase (90 min), anaphase (110 min) and two-cell stages (140 min). The zygotes and two-cell embryos at the above indicated time points were used for immunofluorescence.Citation55
Taxol and nocodazole treatment of oocytes
For experiments in which oocytes were treated with taxol, 5 mM taxol in DMSO stock was diluted in M2 medium to obtain a final concentration of 10 μM and oocytes that were at MI and MII stages were cultured for 50 min; for experiments in which oocytes were treated with nocodazole, 10 mg/ml nocodazole in DMSO stock was diluted in M2 medium to obtain a final concentration of 20 μg/ml and oocytes at MI and MII stages were incubated for 10 min. After treatment, oocytes were washed thoroughly and used for immunofluorescent staining. Oocytes in the control group were treated with the same concentration of DMSO in the medium before examination.
Immunofluorescence and confocal microscopy
Oocytes were fixed with 4% paraformaldehyde/PBS (pH 7.4) for 30 min. After being permeabilized with 0.5% Triton X-100 at room temperature for 20 min, oocytes were blocked in 1% BSA supplemented PBS for 1 h and then incubated with rabbit polyclonal anti-Nek9 antibody(Bioworld;1:50), mouse polyclonal anti-α-tubulin-FITC (Sigma; 1:100) or rabbit polyclonal anti-Bub3 antibody (Santa Cruz Biotechnology; 1:50), respectively, overnight at 4°C. After three washes in washing buffer (0.1% Tween 20 and 0.01% Triton X-100 in PBS) for 5 min each, the oocytes were labeled with FITC-conjugated goat-anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc.; 1:100) for 1 h at room temperature and then washed three times with washing buffer. The oocytes were co-stained with propidium iodide (PI) for 15 min.
For double-staining of Nek9 and α-tubulin, after staining of Nek9, [the secondary antibody was TRITC conjugated goat-anti-rabbit IgG (Jackson ImmunoResearch Laboratories,Inc.; 1:100)] and three washes in washing buffer for 5 min each, oocytes or zygotes were again blocked in blocking buffer (5% normal mouse serum) for 1 h at room temperature, then stained with mouse polyclonal anti-α-tubulin-FITC (Sigma; 1:100) for 2 h at room temperature and, after three washes in washing buffer for 5 min each, were co-stained with Hoechst 33342 for 25 min.
For double staining of Nek9 and γ-tubulin, after staining of Nek9 (the secondary antibody was 1:100 FITC conjugated goat-anti-rabbit IgG) and three washes in washing buffer for 5 min each, oocytes were again blocked in blocking buffer for 1 h at room temperature and then processed using the method described above. Oocytes were stained with 1:100 mouse monoclonal anti-γ-tubulin antibody overnight at 4°C, then after three washes in washing buffer for 5 min each, co-stained with CY5-conjugated goat anti-mouse IgG (Cat#115-175-062 Jackson ImmunoResearch Laboratories,Inc.; 1:100) for 1 h. Finally, oocytes were stained with PI for 15 min.
For triple-staining of Nek9, γ-tubulin and α-tubulin, the methods applied were the same as described above. The second antibodies for Nek9 and γ-tubulin were TRITC conjugated and CY5-conjugated, respectively. DNA was stained with Hoechst 33342 for 25 min.
Zygotes or two-cell embryos Immunofluorescence for Nek9 was performed using the same methods as described above for oocytes.
Finally, oocytes, zygotes or two-cell embryos were mounted on glass slides with anti-fade mounting medium (DABCO) to retard photobleaching and examined with a laser-scanning confocal microscope (Zeiss LSM 780 META).
Chromosome spreading
For chromosome spreading, oocytes were placed in 1% sodium citrate for 10 min at room temperature and then placed on a glass slide. About 100 µl methanol: glacial acetic acid (3:1) was dropped onto the oocyte to break and fix it and 10 μg/ml Hoechst 33342 was used for chromosome staining. The specimens were examined with a laser-scanning confocal microscope (Zeiss LSM 780 META).
Immunoblotting analysis
Immunoblotting was performed as described previously.Citation56 Briefly, 250 mouse oocytes were collected in SDS sample buffer and heated for 5 min at 100°C. The proteins were separated by SDS-PAGE and then electrically transferred to polyvinylidene fluoride membranes. Following transfer, the membranes were blocked in TBST (0.1% Tween 20 diluted in TBS) containing 5% skimmed milk for 2 h, followed by incubation overnight at 4°C with rabbit polyclonal anti-Nek9 antibody (1:1,000) and mouse monoclonal anti-β-actin antibody (1:1,000). After washing three times in TBST, 10 min each, the membranes were incubated for 1 h at 37°C with peroxidase-conjugated goat anti-rabbit IgG (1:1,000) and peroxidase-conjugated rabbit anti-mouse IgG, respectively. Finally, the membranes were processed using the enhanced chemiluminescence-detection system (Amersham).
Microinjection of Nek9 or control morpholino antisense oligos MO and coinjection of β5-tubulin-GFP mRNA with Nek9 or control MO
Microinjections were performed using a Nikon Diaphot ECLIPSE TE 300 (Nikon UK Ltd.) and finished within 30 min. A volume of 2 mM Nek9 MO (GENE TOOLS, LLC, 5′-CGT ACT CGC CCA GCA CCG ACA TGG C-3′) was microinjected into the cytoplasm to delete Nek9. The same amount of negative-control MO (GENE TOOLS, LLC, 5′-CCT CTT ACC TCA GTT ACA ATT TAT A-3′) was also injected as control. To examine how Nek9 knockdown perturbed meiotic division, we co-injected β5-tubulin-GFP mRNA, synthesized as previously reported by Verlhac,Citation57 with 4 mM Nek9 MO or control MO into GV oocytes as described above. Each oocyte was injected with about 10 pl Nek9 MO, control MO or β5-tubulin-GFP mRNA with Nek9MO or β5-tubulin-GFP mRNA with control MO. After microinjection, the oocytes were maintained at the GV stage for 24 h in M2 medium containing 2.5 µM milrinone (SIGMA,Cat#M4659), then thoroughly washed and transferred to milrinone-free M2 medium for further culture, or to M2 containing 10 nM of Hoechst 33342 dye and allowed to continue meiotic maturation. Each experiment consisted of three separate replicates, and approximately 300 oocytes were injected in each group.
Time-lapse live-imaging experiments
Microtubule and chromosome dynamics were filmed on a Perkin Elmer precisely Ultra VIEW VOX confocal Imaging System. A narrow band pass EGFP filter set and a 30% cut neutral density filter from Chroma were used. Exposure time was set ranging between 300–700 ms depending on the tubulin-GFP fluorescence level. The acquisition of digital time-lapse image was controlled by IP Lab (Scanalytics) or AQM6 (Andor/Kinetic-imaging) software packages. Confocal images of spindles and chromosomes in live oocytes were acquired with a 10x oil objective on a spinning disk confocal microscope (Perkin Elmer).
Data analysis
For each treatment, at least three replicates were performed. All percentages from at least three repeated experiments were expressed as means ± SEM, and the number of oocytes observed was labeled in parentheses as (n =). Statistical analyses were conducted by analysis of variance. Differences between treated groups were analyzed by Student-Newman-Keuls test, and p < 0.05 was considered significant.
| Abbreviations: | ||
| Bub1 | = | budding uninhibited by benzimidazole-1 |
| CDK | = | cyclin-dependent kinases |
| COC | = | cumulus-oocyte complexes |
| GV | = | germinal vesicle |
| GVBD | = | germinal vesicle breakdown |
| hCG | = | human chorionic gonadotropin |
| KSOM | = | potassium simplex optimized medium |
| Mad1 | = | mitotic arrest-deficient-1 |
| MI | = | metaphase I |
| MII | = | metaphase II |
| MO | = | morpholino |
| Mps1 | = | monopolar spindle1 |
| MTOCs | = | microtubule-organizing centers |
| Neks | = | NIMA-like kinases |
| NEBD | = | nuclear envelope breakdown |
| NIMA | = | never in mitosis Aspergillus |
| PCM | = | pericentriolar material |
| PMSG | = | pregnant mare serum gonadotropin |
| NEDD1 | = | neural precursor cell expressed developmentally down-regulated protein1 |
| PLK | = | Polo-like kinase |
| PN | = | pronuclei |
| TelI | = | telophase I |
| PB1 | = | first polar body |
| SAC | = | spindle assembly checkpoint |
Additional material
Download Zip (1,008.8 KB)Acknowledgments
We are grateful to Shi-Wen Li, Yi Hou, Li-Juan Wang, Yin-Chun Ouyang and Hua Qin for their technical assistance. This study was supported by the National Basic Research Program of China (2012CB944404, 2011CB944501) and National Natural Science Foundation of China (No.30930065) to Q.Y.S. and by Guang Dong Foshan city Technology project (No.0005907120416038) to S.W.Y.
Note
Time-lapse live-cell image videos are given to support the role of Nek9 in meiotic maturation. Oocytes were placed onto the confocal Imaging System at 3 h of release from M2 medium containing 2.5 µM milrinone. In Figure S1, control oocyte was observed with normal spindle morphology and well-aligned chromosomes and completed first polar body extrusion at 14 h. In contrast, Figure S2 shows Nek9-MO oocyte with abnormal spindles, aberrant chromosomes and failure to extrude PB1 even at 16 h of culture. Tubulin (green); DNA (red).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Niikura Y, Niikura T, Tilly JL. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging (Albany NY) 2009; 1:971 - 8; PMID: 20157580
- Niikura Y, Niikura T, Wang N, Satirapod C, Tilly JL. Systemic signals in aged males exert potent rejuvenating effects on the ovarian follicle reserve in mammalian females. Aging (Albany NY) 2010; 2:999 - 1003; PMID: 21212462
- Homer H, Gui L, Carroll J. A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science 2009; 326:991 - 4; http://dx.doi.org/10.1126/science.1175326; PMID: 19965510
- Fry AM. The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene 2002; 21:6184 - 94; http://dx.doi.org/10.1038/sj.onc.1205711; PMID: 12214248
- Carabatsos MJ, Combelles CM, Messinger SM, Albertini DF. Sorting and reorganization of centrosomes during oocyte maturation in the mouse. Microsc Res Tech 2000; 49:435 - 44; http://dx.doi.org/10.1002/(SICI)1097-0029(20000601)49:5<435::AID-JEMT5>3.0.CO;2-H; PMID: 10842370
- Ma W, Koch JA, Viveiros MM. Protein kinase C delta (PKCdelta) interacts with microtubule organizing center (MTOC)-associated proteins and participates in meiotic spindle organization. Dev Biol 2008; 320:414 - 25; http://dx.doi.org/10.1016/j.ydbio.2008.05.550; PMID: 18602096
- Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 2007; 130:484 - 98; http://dx.doi.org/10.1016/j.cell.2007.06.025; PMID: 17693257
- Dumont J, Petri S, Pellegrin F, Terret ME, Bohnsack MT, Rassinier P, et al. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J Cell Biol 2007; 176:295 - 305; http://dx.doi.org/10.1083/jcb.200605199; PMID: 17261848
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol 2001; 2:21 - 32; http://dx.doi.org/10.1038/35048096; PMID: 11413462
- McMillin DW, Delmore J, Negri J, Ooi M, Klippel S, Miduturu CV, et al. Microenvironmental influence on pre-clinical activity of polo-like kinase inhibition in multiple myeloma: implications for clinical translation. PLoS One 2011; 6:e20226; http://dx.doi.org/10.1371/journal.pone.0020226; PMID: 21750699
- Luo J, Liu X. Polo-like kinase 1, on the rise from cell cycle regulation to prostate cancer development. Protein Cell 2012; 3:182 - 97; http://dx.doi.org/10.1007/s13238-012-2020-y; PMID: 22447658
- van der Waal MS, Hengeveld RC, van der Horst A, Lens SM. Cell division control by the Chromosomal Passenger Complex. Exp Cell Res 2012; 318:1407 - 20; http://dx.doi.org/10.1016/j.yexcr.2012.03.015; PMID: 22472345
- Hégarat N, Smith E, Nayak G, Takeda S, Eyers PA, Hochegger H. Aurora A and Aurora B jointly coordinate chromosome segregation and anaphase microtubule dynamics. J Cell Biol 2011; 195:1103 - 13; http://dx.doi.org/10.1083/jcb.201105058; PMID: 22184196
- Salaun P, Rannou Y, Prigent C. Cdk1, Plks, Auroras, and Neks: the mitotic bodyguards. Adv Exp Med Biol 2008; 617:41 - 56; http://dx.doi.org/10.1007/978-0-387-69080-3_4; PMID: 18497029
- Osmani SA, Ye XS. Cell cycle regulation in Aspergillus by two protein kinases. Biochem J 1996; 317:633 - 41; PMID: 8760343
- O’Connell MJ, Krien MJ, Hunter T. Never say never. The NIMA-related protein kinases in mitotic control. Trends Cell Biol 2003; 13:221 - 8; http://dx.doi.org/10.1016/S0962-8924(03)00056-4; PMID: 12742165
- Rapley J, Nicolàs M, Groen A, Regué L, Bertran MT, Caelles C, et al. The NIMA-family kinase Nek6 phosphorylates the kinesin Eg5 at a novel site necessary for mitotic spindle formation. J Cell Sci 2008; 121:3912 - 21; http://dx.doi.org/10.1242/jcs.035360; PMID: 19001501
- Wu L, Osmani SA, Mirabito PM. A role for NIMA in the nuclear localization of cyclin B in Aspergillus nidulans. J Cell Biol 1998; 141:1575 - 87; http://dx.doi.org/10.1083/jcb.141.7.1575; PMID: 9647650
- Quarmby LM, Mahjoub MR. Caught Nek-ing: cilia and centrioles. J Cell Sci 2005; 118:5161 - 9; http://dx.doi.org/10.1242/jcs.02681; PMID: 16280549
- O’regan L, Blot J, Fry AM. Mitotic regulation by NIMA-related kinases. Cell Div 2007; 2:25; http://dx.doi.org/10.1186/1747-1028-2-25; PMID: 17727698
- Bowers AJ, Boylan JF. Nek8, a NIMA family kinase member, is overexpressed in primary human breast tumors. Gene 2004; 328:135 - 42; http://dx.doi.org/10.1016/j.gene.2003.12.002; PMID: 15019993
- Roig J, Mikhailov A, Belham C, Avruch J. Nercc1, a mammalian NIMA-family kinase, binds the Ran GTPase and regulates mitotic progression. Genes Dev 2002; 16:1640 - 58; http://dx.doi.org/10.1101/gad.972202; PMID: 12101123
- Roig J, Groen A, Caldwell J, Avruch J. Active Nercc1 protein kinase concentrates at centrosomes early in mitosis and is necessary for proper spindle assembly. Mol Biol Cell 2005; 16:4827 - 40; http://dx.doi.org/10.1091/mbc.E05-04-0315; PMID: 16079175
- Belham C, Roig J, Caldwell JA, Aoyama Y, Kemp BE, Comb M, et al. A mitotic cascade of NIMA family kinases. Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J Biol Chem 2003; 278:34897 - 909; http://dx.doi.org/10.1074/jbc.M303663200; PMID: 12840024
- O’Regan L, Fry AM. The Nek6 and Nek7 protein kinases are required for robust mitotic spindle formation and cytokinesis. Mol Cell Biol 2009; 29:3975 - 90; http://dx.doi.org/10.1128/MCB.01867-08; PMID: 19414596
- Bertran MT, Sdelci S, Regué L, Avruch J, Caelles C, Roig J. Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. EMBO J 2011; 30:2634 - 47; http://dx.doi.org/10.1038/emboj.2011.179; PMID: 21642957
- Sonn S, Oh GT, Rhee K. Nek2 and its substrate, centrobin/Nip2, are required for proper meiotic spindle formation of the mouse oocytes. Zygote 2011; 19:15 - 20; http://dx.doi.org/10.1017/S0967199410000183; PMID: 20569513
- Meng XQ, Fan HY, Zhong ZS, Zhang G, Li YL, Chen DY, et al. Localization of gamma-tubulin in mouse eggs during meiotic maturation, fertilization, and early embryonic development. J Reprod Dev 2004; 50:97 - 105; http://dx.doi.org/10.1262/jrd.50.97; PMID: 15007207
- Xiong B, Sun SC, Lin SL, Li M, Xu BZ, OuYang YC, et al. Involvement of Polo-like kinase 1 in MEK1/2-regulated spindle formation during mouse oocyte meiosis. Cell Cycle 2008; 7:1804 - 9; http://dx.doi.org/10.4161/cc.7.12.6019; PMID: 18583944
- Yan LY, Huang JC, Zhu ZY, Lei ZL, Shi LH, Nan CL, et al. NuMA distribution and microtubule configuration in rabbit oocytes and cloned embryos. Reproduction 2006; 132:869 - 76; http://dx.doi.org/10.1530/rep.1.01224; PMID: 17127747
- Ou XH, Li S, Xu BZ, Wang ZB, Quan S, Li M, et al. p38α MAPK is a MTOC-associated protein regulating spindle assembly, spindle length and accurate chromosome segregation during mouse oocyte meiotic maturation. Cell Cycle 2010; 9:4130 - 43; http://dx.doi.org/10.4161/cc.9.20.13389; PMID: 20948319
- Zhang CH, Wang ZB, Quan S, Huang X, Tong JS, Ma JY, et al. GM130, a cis-Golgi protein, regulates meiotic spindle assembly and asymmetric division in mouse oocyte. Cell Cycle 2011; 10:1861 - 70; http://dx.doi.org/10.4161/cc.10.11.15797; PMID: 21552007
- Nigg EA. Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol 1998; 10:776 - 83; http://dx.doi.org/10.1016/S0955-0674(98)80121-X; PMID: 9914175
- Tong C, Fan HY, Lian L, Li SW, Chen DY, Schatten H, et al. Polo-like kinase-1 is a pivotal regulator of microtubule assembly during mouse oocyte meiotic maturation, fertilization, and early embryonic mitosis. Biol Reprod 2002; 67:546 - 54; http://dx.doi.org/10.1095/biolreprod67.2.546; PMID: 12135894
- Kim S, Lee K, Rhee K. NEK7 is a centrosomal kinase critical for microtubule nucleation. Biochem Biophys Res Commun 2007; 360:56 - 62; http://dx.doi.org/10.1016/j.bbrc.2007.05.206; PMID: 17586473
- Yin MJ, Shao L, Voehringer D, Smeal T, Jallal B. The serine/threonine kinase Nek6 is required for cell cycle progression through mitosis. J Biol Chem 2003; 278:52454 - 60; http://dx.doi.org/10.1074/jbc.M308080200; PMID: 14563848
- Wiese C, Zheng Y. Microtubule nucleation: gamma-tubulin and beyond. J Cell Sci 2006; 119:4143 - 53; http://dx.doi.org/10.1242/jcs.03226; PMID: 17038541
- Ma W, Baumann C, Viveiros MM. NEDD1 is crucial for meiotic spindle stability and accurate chromosome segregation in mammalian oocytes. Dev Biol 2010; 339:439 - 50; http://dx.doi.org/10.1016/j.ydbio.2010.01.009; PMID: 20079731
- Haren L, Remy MH, Bazin I, Callebaut I, Wright M, Merdes A. NEDD1-dependent recruitment of the gamma-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J Cell Biol 2006; 172:505 - 15; http://dx.doi.org/10.1083/jcb.200510028; PMID: 16461362
- Haren L, Stearns T, Lüders J. Plk1-dependent recruitment of gamma-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS One 2009; 4:e5976; http://dx.doi.org/10.1371/journal.pone.0005976; PMID: 19543530
- Sdelci S, Schütz M, Pinyol R, Bertran MT, Regué L, Caelles C, et al. Nek9 phosphorylation of NEDD1/GCP-WD contributes to Plk1 control of γ-tubulin recruitment to the mitotic centrosome. Curr Biol 2012; 22:1516 - 23; http://dx.doi.org/10.1016/j.cub.2012.06.027; PMID: 22818914
- Kalab P, Heald R. The RanGTP gradient - a GPS for the mitotic spindle. J Cell Sci 2008; 121:1577 - 86; http://dx.doi.org/10.1242/jcs.005959; PMID: 18469014
- Clarke PR, Zhang C. Spatial and temporal coordination of mitosis by Ran GTPase. Nat Rev Mol Cell Biol 2008; 9:464 - 77; http://dx.doi.org/10.1038/nrm2410; PMID: 18478030
- Kaláb P, Solc P, Motlík J. The role of RanGTP gradient in vertebrate oocyte maturation. Results Probl Cell Differ 2011; 53:235 - 67; http://dx.doi.org/10.1007/978-3-642-19065-0_12; PMID: 21630149
- Wei L, Liang XW, Zhang QH, Li M, Yuan J, Li S, et al. BubR1 is a spindle assembly checkpoint protein regulating meiotic cell cycle progression of mouse oocyte. Cell Cycle 2010; 9:1112 - 21; http://dx.doi.org/10.4161/cc.9.6.10957; PMID: 20237433
- Li M, Li S, Yuan J, Wang ZB, Sun SC, Schatten H, et al. Bub3 is a spindle assembly checkpoint protein regulating chromosome segregation during mouse oocyte meiosis. PLoS One 2009; 4:e7701; http://dx.doi.org/10.1371/journal.pone.0007701; PMID: 19888327
- Althoff F, Karess RE, Lehner CF. Spindle checkpoint-independent inhibition of mitotic chromosome segregation by Drosophila Mps1. Mol Biol Cell 2012; 23:2275 - 91; http://dx.doi.org/10.1091/mbc.E12-02-0117; PMID: 22553353
- Hached K, Xie SZ, Buffin E, Cladière D, Rachez C, Sacras M, et al. Mps1 at kinetochores is essential for female mouse meiosis I. Development 2011; 138:2261 - 71; http://dx.doi.org/10.1242/dev.061317; PMID: 21558374
- Homer HA. Mad2 and spindle assembly checkpoint function during meiosis I in mammalian oocytes. Histol Histopathol 2006; 21:873 - 86; PMID: 16691540
- Bannon JH, O’Donovan DS, Kennelly SM, Mc Gee MM. The peptidyl prolyl isomerase cyclophilin A localizes at the centrosome and the midbody and is required for cytokinesis. Cell Cycle 2012; 11:1340 - 53; http://dx.doi.org/10.4161/cc.19711; PMID: 22421161
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The active form of the metabolic sensor: AMP-activated protein kinase (AMPK) directly binds the mitotic apparatus and travels from centrosomes to the spindle midzone during mitosis and cytokinesis. Cell Cycle 2009; 8:2385 - 98; http://dx.doi.org/10.4161/cc.8.15.9082; PMID: 19556893
- Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Menendez JA. Polo-like kinase 1 regulates activation of AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell Cycle 2011; 10:1295 - 302; http://dx.doi.org/10.4161/cc.10.8.15342; PMID: 21474997
- Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Menendez JA. Polo-like kinase 1 directs the AMPK-mediated activation of myosin regulatory light chain at the cytokinetic cleavage furrow independently of energy balance. Cell Cycle 2012; 11:2422 - 6; http://dx.doi.org/10.4161/cc.20438; PMID: 22672936
- Li J, Wang J, Hou W, Jing Z, Tian C, Han Y, et al. Phosphorylation of Ataxin-10 by polo-like kinase 1 is required for cytokinesis. Cell Cycle 2011; 10:2946 - 58; http://dx.doi.org/10.4161/cc.10.17.15922; PMID: 21857149
- Wei Y, Multi S, Yang CR, Ma J, Zhang QH, Wang ZB, et al. Spindle assembly checkpoint regulates mitotic cell cycle progression during preimplantation embryo development. PLoS One 2011; 6:e21557; http://dx.doi.org/10.1371/journal.pone.0021557; PMID: 21720555
- Sun SC, Wei L, Li M, Lin SL, Xu BZ, Liang XW, et al. Perturbation of survivin expression affects chromosome alignment and spindle checkpoint in mouse oocyte meiotic maturation. Cell Cycle 2009; 8:3365 - 72; http://dx.doi.org/10.4161/cc.8.20.9855; PMID: 19806029
- Verlhac MH, Lefebvre C, Guillaud P, Rassinier P, Maro B. Asymmetric division in mouse oocytes: with or without Mos. Curr Biol 2000; 10:1303 - 6; http://dx.doi.org/10.1016/S0960-9822(00)00753-3; PMID: 11069114