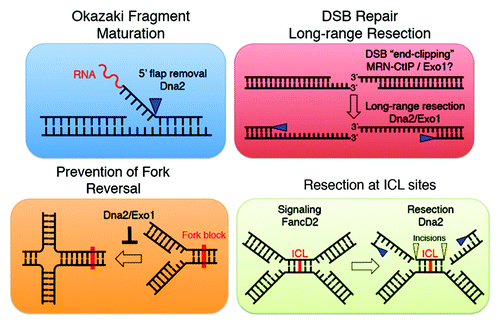
Dna2 was first characterized in yeast as an essential gene encoding a protein with both helicase and endonuclease activities involved in maturation of Okazaki fragments during DNA replication. Dna2 also plays a role in double-strand break (DSB) repair by homologous recombination. The respective contributions of its replication and/or repair functions toward cell viability and resistance to genotoxic stress is not entirely clear. Recent studies, including that of Karanja et al.Citation1 in a recent issue of Cell Cycle, are starting to clarify the multifaceted roles of DNA2.
Together with the endonuclease Rad27 (Fen1 in higher eukaryotes), Dna2 removes 5′ flaps generated by strand displacement during synthesis by Pol δ on the lagging strand. Most 5′ flap processing during replication is due to the activity of Rad27, yet Rad27∆ yeast cells are viable, whereas Dna2∆ cells are not. This suggests that the essential role of Dna2 in genome maintenance is distinct from Okazaki fragment maturation.Citation2 Indeed, Dna2 is a target of the intra-S-phase checkpoint in fission yeast and stabilizes replication forks.Citation3 Dna2 phosphorylation by Cds1 promotes the association of Dna2 to replication forks to counteract fork reversal. Reversed forks can be erroneously recognized as recombination intermediates leading genomic rearrangements.Citation4 Thus, Dna2 maintains genome stability by processing stalled forks before they collapse into aberrant structures. Similarly, Exo1 nuclease also participates in preventing the generation of “chicken-foot” structures from blocked forks, but, interestingly, Exo1 appears to be functional, even in the absence of an active checkpoint.Citation4
Homology-dependent repair requires the generation of 3′ ssDNA, a process called resection that is regulated by CDKs. Resection provides the template that is used by Rad51 recombinase to search for homologous sequences.Citation5 Resection is initiated by the MRN (Mre11-Rad50-Nbs1) complex and its co-factor CtIP. More processive, long-range resection is then performed by two partially overlapping pathways involving Dna2 and/or Exo1.Citation5
In the November 2012 issue of Cell Cycle, Karanja et al.Citation1 provide additional evidence for the role of Dna2 and Exo1 in S-phase. Using siRNA-mediated Dna2 and Exo1 knockdown, they show that both nucleases contribute to cell viability following CPT, cisplatin or MMS treatments in a redundant manner. The data further support the conserved roles of Dna2 and Exo1 in the DNA damage response. Notably, the authors observe that Dna2-depleted cells have more profound defects in resection and Chk1 activation than Exo1-depleted cells when treated with cisplatin, a DNA-damaging agent that generates DNA interstrand cross-links (ICLs).
The Fanconi anemia/BRCA (FA/BRCA) pathway is critical for ICL repair in proliferating cells.Citation6 Recent work in the Xenopus system showed that the FA/BRCA pathway modulates the DNA damage response to ICLs and promotes ICL repair during S-phase.Citation7,Citation8 Upon stalling at an ICL, the Fanconi anemia pathway promotes both stabilization of replication forks and recruitment of structure-specific nucleases to perform incision on both sides of the ICL. Rad51 loading takes place at the lesion before a DSB is generated,Citation9 suggesting that resection initiates from a partially processed ICL. Then translesion DNA synthesis is performed across the ICL site, the adduct is removed and the fork is most likely re-established by HDR. The mechanism of the resection step and the nature of the nucleases involved are still unknown, but given that Dna2 is present at replication forks, that it is involved in resection at DSBs and that it is regulated by the S-phase checkpoint, makes it an attractive candidate to perform this task. Notably, Karanja et al. detect a physical interaction between Dna2 and FancD2. Furthermore, experiments in FancD2-null cells show that Dna2 works downstream or parallel to FancD2, suggesting a function for Dna2 in ICL processing. FancD2 complex participates in signaling from ICL damage and in recruiting incision nucleases to the lesions, functions that could both involve Dna2. Nevertheless, it is also conceivable that Dna2 helps to prevent fork regression during ICL repair.
In summary, these findings further position Dna2 as a versatile checkpoint-regulated nuclease working during chromosomal replication and repair and essential for maintaining genome stability. Further studies are needed to understand the precise role of resection during ICL repair and the role of Dna2 and Exo1 nucleases, which appear to be partially redundant in this process.
References
- Karanja KK, et al. Cell Cycle 2012; 11:3983 - 96; http://dx.doi.org/10.4161/cc.22215; PMID: 22987153
- Budd ME, et al. Mutat Res 2000; 459:173 - 86; http://dx.doi.org/10.1016/S0921-8777(99)00072-5; PMID: 10812329
- Hu J, et al. Cell 2012; 149:1221 - 32; http://dx.doi.org/10.1016/j.cell.2012.04.030; PMID: 22682245
- Branzei D, et al. Nat Rev Mol Cell Biol 2010; 11:208 - 19; http://dx.doi.org/10.1038/nrm2852; PMID: 20177396
- Symington LS, et al. Annu Rev Genet 2011; 45:247 - 71; http://dx.doi.org/10.1146/annurev-genet-110410-132435; PMID: 21910633
- Deans AJ, et al. Nat Rev Cancer 2011; 11:467 - 80; http://dx.doi.org/10.1038/nrc3088; PMID: 21701511
- Ben-Yehoyada M, et al. Mol Cell 2009; 35:704 - 15; http://dx.doi.org/10.1016/j.molcel.2009.08.014; PMID: 19748363
- Knipscheer P, et al. Science 2009; 326:1698 - 701; http://dx.doi.org/10.1126/science.1182372; PMID: 19965384
- Long DT, et al. Science 2011; 333:84 - 7; http://dx.doi.org/10.1126/science.1204258; PMID: 21719678