Abstract
Loss of function in either VHL or Nek1 leads to cyst formation in tissues, especially in kidneys. Whether there is a connection between pVHL and Nek1 regulation is unknown. Here, we report that the VHL protein (pVHL) may be a substrate of Nek1. While Nek1 can phosphorylate pVHL at multiple sites, the phosphorylation at serine-168 results in pVHL degradation. Nek1-mediated phosphorylation of pVHL does not significantly affect hypoxia-inducible factors (HIF), a known target of pVHL. However, non-phosphorylable pVHL reconstituted in VHL-deficient cells induces more stable cilia than wild-type VHL during serum stimulation and Nocodazole treatment. The results suggest a possible regulation of pVHL by Nek1 that may contribute to ciliary homeostasis and cystogenesis.
Introduction
Von Hippel-Lindau protein (pVHL) is a tumor suppressor, mutation of which predisposes to a variety of malignant and benign tumors including renal cell carcinoma, the major type of kidney cancer.Citation1-Citation3 Pathological features of VHL deletion or loss of function include blood vessel expansion and the formation of cysts in affected tissues. Mechanistically, VHL is well-recognized for its regulation of hypoxia-inducible factors (HIF). As an E3 ligase, VHL mediates the ubiqitination of HIF to target it for proteasomal degradation.Citation4-Citation6 However, VHL has HIF-independent actions.Citation7 For example, VHL has been shown to modulate cell matrix.Citation8,Citation9 More recent work suggests that VHL also participates in the regulation of primary cilium via the stabilization of microtubules.Citation10,Citation11 Despite these findings, the regulation of VHL itself is largely unknown.
Nek kinases are mammalian homologs of NIMA, never in mitosis gene A in Aspergillus nidulans. Acting mainly as serine/threonine protein kinases, Neks participate in a variety of cellular processes. Nek2, 6, 7 and 9 play important roles in the regulation of mitotic progression, including chromatin condensation, spindle organization and cytokinesis. Nek1 and 8 have been implicated in the regulation of cilia.Citation12-Citation15 Nek1 has been shown to function in DNA damage response to induce cell cycle arrest in a novel, ATM/ATR-independent pathway.Citation16,Citation17 In mice, the loss-of-function mutation of Nek1 results in the development of cystic kidneys with the characteristics of autosomal dominant polycystic kidney disease.Citation18 In humans, Nek1 mutations have been suggested to be a key causative factor in short-rib polydactyly syndrome, Majewski type, which is characterized by malformations in brain, polydactylity and kidney cysts.Citation19 Despite such remarkable phenotypes, the mechanism whereby Nek1 mutation induces the pathologies is largely unknown. Interestingly, as pVHL, Nek1 may also participate in the regulation of cilia. Shalom and colleagues reported that Nek1 overexpression leads to cilia resorption in cultured cells, and the cells from Nek1 mutant mice have defective cilia,Citation13,Citation20 although the underlying mechanism remains elusive.
Considering the fact that both pVHL and Nek1 play a role in ciliary regulation and cyst formation, we postulated a molecular connection between these two signaling molecules. In this study, we have tested the possibility that Nek1 may phosphorylate pVHL, contributing to the maintenance of ciliary homeostasis. We report that Nek1 may phosphorylate pVHL at multiple sites and promote pVHL degradation. Mutation of pVHL at serine-168, a Nek1 phosphorylation site, increases the stability of pVHL and improves its capacity to stabilize cilia.
Results
Overexpression of Nek1 induces pVHL degradation
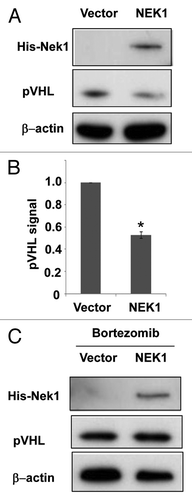
To determine a possible regulation of pVHL by Nek1, we first examined the effect of Nek1 overexpression on pVHL in HEK cells. Compared with empty vector, the transfection of Nek1 led to a noticeable decrease of pVHL (). The result was verified by densitometry analysis of the immunoblots (). Interestingly, the proteasome inhibitor Bortezomib could recover pVHL in Nek1-tranfected cells to a level comparable to that of vector-transfected cells (). The results suggest that Nek1 promotes protoesomomal degradation of pVHL. The binding of elongin B and C may stabilize VHL from proteasomal degradation.Citation21 We thought that Nek1 may promote pVHL degradation by preventing elongin B/C binding to pVHL. To address this, HA-VHL was transfected into HEK cells for co-immunoprecipitation (co-IP) analysis. As shown in Figure S1, Nek1 expression did not significantly change HA-pVHL/elongin B co-IP. Thus, Nek1 may induce proteasomal degradation of pVHL by elongin binding-independent mechanisms.
Figure 1. Overexpression of Nek1 leads to pVHL degradation. (A) HEK cells were transfected with Nek1-pcDNA3.1-Myc/His or empty vector to collect whole-cell lysate 36 h later for immunoblot analysis of pVHL, Nek1-Myc/His and β-actin. (B) Densitometric analysis of immunoblots. pVHL signal was normalized by β-actin signal in the immunblots from three experiments. (C) HEK cells were transfected with Nek1-pcDNA3.1-Myc/His or empty vector for 36 h and then treated with 100 nm Bortezomib for 4 h to collect whole-cell lysate for immunoblot analysis of VHL, Nek1-Myc/His and β-actin.
Nek1 interacts and phosphorylates pVHL
Although Nek kinases mainly phosphorylate their substrates at serine/threonine sites, Nek1 may have dual specificity.Citation15 Bioinformatic analysis indicated that pVHL contains multiple putative Nek phosphorylation sites. To test the possibility that Nek1 may phosphorylate pVHL, we initially examined the interaction between these two proteins. To this end, HEK cells were co-transfected with Nek1-Myc/His and HA-VHL for co-IP. As shown in , anti-HA antibody pulled down both HA-pVHL and Nek1-Myc/His. Endogenous Nek1 was not revealed in the immunoprecipitates, probably due to the low level of expression. To determine if Nek1 can phosphorylate pVHL, we immunoprecipitated Nek1-Myc/His from transfected cells and added it to recombinant pVHL in the presence of (γ-32P)ATP. As shown in , Nek1-Myc/His immunoprecipitate induced significantly higher pVHL phosphorylation than the immunoprecipate from vector-tranfected cells. To determine if Nek1 can directly phosphorylate pVHL, recombinant active Nek1 and pVHL were incubated separately or together in the presence of (γ-32P)ATP. As shown in , the addition of Nek1 induced a marked phosphorylation of pVHL. Nek1 also showed significant autophosphorylation in this in vitro assay (). To identify the phosphorylated sites in pVHL, recombinant Nek1 and pVHL were incubated and resolved on SDS-PAGE for Coomassie blue staining. The VHL band was collected for phospho-peptide analysis by mass spectrometry. The analysis identified seven phosphorylated sites, including threonine-100, threonine-105, tyrosine-111, serine-168, serine-183, tyrosine-175, serine-183 and threonine-202 ().
Figure 2. Nek1 interacts with and phosphorylates pVHL. (A) HEK 293 cells were co transfected with Nek1-pcDNA3.1-Myc/His and HA-VHL-pRc/CMV to collect whole-cell lysate 36 h for immunoprecipitation using anti-HA antibody, followed by immunoblot analysis using anti-Myc and anti-HA antibodies. (B) HEK cells were transfected with Nek1-pcDNA3.1-Myc/His or empty vector to collect cell lysate 36 h later for immunoprecipitation of Nek1-Myc/His. The immunoprecipitates were incubated with recombinant pVHL in the presence of (γ-32P)ATP. The samples were then resolved by gel electrophoresis, followed by autoradiography. (C) Purified recombinant active Nek1 and pVHL were incubated separately or together in kinase reaction buffer with (γ-32P)ATP. The samples were then resolved by gel electrophoresis, followed by autoradiography. (D) purified recombinant Nek1 and pVHL were incubated in kinase reaction buffer, followed by gel electrophoresis and Commassie Blue staining. The pVHL band was exercised for mass spectrometry analysis of phosphorylated sites (highlighted in red at indicated sites).
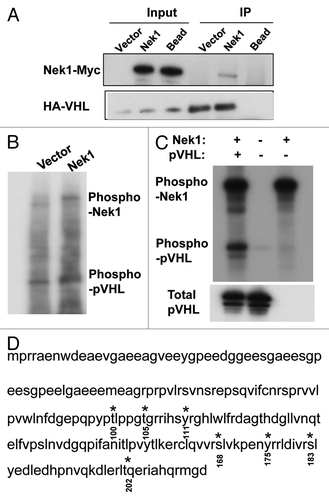
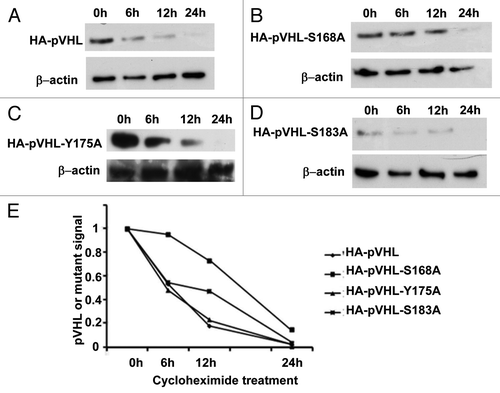
Figure 3. Mutation of pVHL at S-168 increases protein stability. HEK cells were transfected with HA-pVHL or its mutants for 36 h. The cells were then treated with cycloheximide to collect lysates at indicated time points for immunoblot analysis of HA-pVHL (A) or its mutants (B–D) using anti-HA antibodies. The results of densitometry are shown in (E).
Mutation of pVHL at serine-168 increases protein stability
Since Nek1 may induce pVHL degradation and phosphorylation ( and ), we hypothesized that phosphorylation of pVHL by Nek1 at specific sites led to pVHL degradation and reduction in its stability. To test this possibility, we generated pVHL mutants at the phosphorylation sites identified above by mass-spectrometry. We particularly focused on the C-terminal S168A, Y175A and S183A mutants, because pVHL stability is largely determined by the C-terminal domain.Citation22 HA-VHL and its mutants were separately transfected into HEK cells, which were then treated with cycloheximide to block new protein synthesis. Cell lysate was collected at various time points after cycloheximide chase for immunoblot analysis of HA-pVHL or its mutants. As shown in , wild-type HA-pVHL was drastically reduced within 6–12 h of cycloheximide chase and completely disappeared in 24 h. Similar results were shown for pVHL-Y175A and S183A mutants (). In contrast, pVHL-S168A mutant was largely preserved in the first 12 h and became notably reduced only after 24 h of cycloheximide chase (). The result of densitometry of the immunoblots is presented in . Clearly, mutation of serine-168, but not serine-183 or tyrosine-175, increased the stability of pVHL.
Nek1 and pVHL-S168A mutation do not affect HIF, but increase the stability of cilia
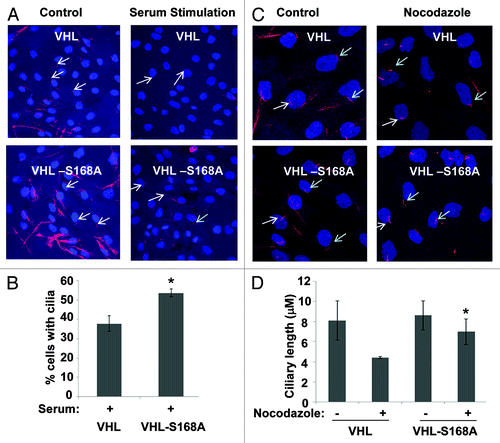
A well-known function of pVHL is to act as an E3 ligase for the ubiquitination of HIFα and consequent proteasome degradation. Since VHL stability was affected by Nek1 overexpression and serine-168 mutation (–), we tested whether these manipulations may affect HIFα expression. Regardless of the transfection of Nek1, HIF-1α was undetectable in HEK cells under normoxia (Fig. S2A). In addition, Nek1 did not significantly affect HIF-1α induction by hypoxia in HEK cells (Fig. S2A). 786-O is a renal cell carcinoma cell line that is deficient in VHL and expresses HIF-2α under normoxia.Citation23 By stable transfection, we generated 786-O cells with wild-type VHL, VHL-S168A or VHL-S168D. As shown in Figure S2B, vector-transfected 786-O cells expressed HIF-2α under normoxia; however, HIF-2α became undetectable after the reconstitution with either wild-type or mutant pVHL, indicating that serine-168 mutation does not alter the regulatory function of pVHL toward HIF. In addition to HIF, VHL may also regulate other proteins and relevant cellular processes. Recent work has suggested that pVHL may stabilize microtubules for the maintenance of cilia.Citation10,Citation11 Therefore, we examined the effects of pVHL and its S168A mutant on ciliary homeostasis. In this experiment, we used RCC4, a renal cell carcinoma cell line that is defective in VHL and ciliogenesis.Citation24 We generated RCC4 cell lines expressing VHL or VHL-S168A mutant by stable transfection. Both VHL-RCC4 and VHL-S168A-RCC4 cells grew cilia, and there were not significant differences in ciliary growth in these two cell lines (). We further analyzed ciliary stability in these cells. To this end, the cells were starved to grow cilia and then subjected to serum stimulation. As shown in , after 24 h of serum simulation, the number of cilia-bearing cells was reduced to 37% in VHL-RCC4 cells; however, 53% of VHL-S168A cells maintained cilia after serum stimulation. Nocodazole disrupts microtubules, resulting in the shortening of cilia.Citation11 In VHL-RCC4 cells, cilia became small and speckle-like after 3 h of nocodazole treatment (), and cilia length was decreased from 8.1 to 4.4 μM (). In contrast, VHL-S168A-RCC4 cells mostly maintained hair-like cilia after nocodazole treatment (), and their cilia were 6.9 μM after nocodazole treatment, significantly longer than VHL-RCC4 cells (). Together, these results suggest that mutation of Serine-168 in pVHL, a possible Nek1 phosphorylation site, may enhance its microtubule stabilizing function to improve the stability of cilia in cells.
Figure 4. pVHL-S168A induces cilia that are more stable than those induced by pVHL. RCC4 cells were stably transfected with VHL or its S168A mutant. The cells were serum-starved for 48 h, followed by 24 h of serum stimulation or 3 h of nocodazole treatment. The cells were fixed for acetyl-tubulin immunofluorescence to evaluate cilia. (A and C) Representative images. Red, acetyl-tubulin staining; blue, nuclei: arrows, representative cilia. (B) Cilia were counted to determine the percentage of cells with cilia after serum stimulation. Data: mean+/−SD, n = 3; * p < 0.05 vs. pVHL-reconstituted cells after serum stimulation. (D) The length of cilia was measured using LSM image analyzer. Data: mean+/−SD, n = 20; * p < 0.01 vs. pVHL-reconstituted cells after nocodazole treatment.
Conclusions
This study has demonstrated evidence for the regulation of pVHL by Nek1. Overexpression of Nek1 led to the decrease in pVHL, which was rescued by Bortezomib, suggesting that Nek1 promotes proteasome degradation of pVHL. Mechanistically, co-IP assay indicated that Nek1 interacts with pVHL, and, notably, in vitro analysis revealed that Nek1 may directly phosphorylate pVHL. Mass spectrometry showed that Nek1 may phosphorylate pVHL at multiple sites, including both serine/threonine and tyrosine residues. Consistently, in the in vitro kinase assay, mutation of a specific site (e.g., Serine-168) did not significantly attenuate Nek1-mediated pVHL phosphorylation (not shown). Due to failure to produce antibodies specific for phosphorylated pVHL (not shown), further evidence for Nek1-mediated phosphorylation of pVHL in vivo has yet to be established. Nevertheless, we showed that the non-phosphorylatable mutation of pVHL at serine-168 results in improved protein stability, suggesting that phosphorylation of pVHL by Nek1 at this site may lead to proteasome degradation. Previous studies demonstrated that pVHL may be subjected to phosphorylation regulation by casein kinase-2, glycogen synthase kinase 3 and checkpoint kinase-2.Citation11,Citation25-Citation28 Phosphorylation of pVHL by these kinases affects pVHL stability and/or its binding to other proteins.Citation11,Citation25-Citation28 Our current results suggest that Nek1 may be a new protein kinase that phosphorylates and regulates pVHL. Functionally, although Nek1 promoted pVHL degradation, it did not affect HIF in normoxic cells or its induction in hypoxic cells, suggesting that the residual pVHL may be sufficient for HIF regulation. Consistently, pVHL-S168A mutant was as effective as wild-type pVHL in targeting HIF for degradation after being reconstituted into 786-O cells, further suggesting that phosphorylation of pVHL by Nek1 may not be a key regulatory mechanism of HIF. On the other hand, pVHL-S168A induced cilia in RCC4 cells that were more stable than those induced by pVHL, suggesting that phosphorylation of pVHL by Nek1 may regulate ciliary homeostasis. Previous studies showed that overexpression of Nek1 inhibits ciliogenesis, but the underlying mechanism is unclear.Citation13,Citation20 Our observations suggest that Nek1 may suppress cilia by phosphorylating pVHL, which is critical to microtubule stabilization and ciliary stability.
Materials and Methods
Reagents
Antibodies for HA, His and Myc tag were from Cell Signaling Technologies (catalog number 3724, 2365, 2278, respectively). Antibodies for β-actin and acetylated tubulin were from Sigma Aldrich (A5441 and T7451). Cycloheximide and Nocodazole were from Sigma Aldrich (C7698 and M1404, respectively). Recombinant purified Nek1 was from Invitrogen, (PV4202) and G418 from Gibco (11811031). Recombinant VHL was from Protein one (P2010). Bortezomib was from LC laboratories (B 1408).
Cell lines and plasmids
HEK cells were purchased from ATCC and grown in MEM supplemented with 10% fetal bovine serum (FBS). 786-O cells were from ATCC and maintained in DMEM supplemented with 10% FBS. RCC4 cells were a kind gift of Dr. Celeste Simon at University of Pennsylvania and maintained in DMEM supplemented with 10% FBS, 1% EAA, 1% glutamine. 786-O and RCC4 cells were transfected with VHL or it mutants and then selected with G418 at 1 mg/ml to generate stable cell lines. Nek1 coding cDNA was purchased from Open Biosystem. The coding region was PCR amplified and cloned in to pcDNA3.1- Myc/His. HA-VHL-pRc-CMV was a kind gift of Dr. William Kaelin at Harvard Medical School. VHL mutants were generated by using QuickChange Mutagenesis Kit (Agilent Technologies, 200519).
Hypoxia
HEK cells transfected with Nek1-Myc/His or vector alone plasmids were treated with hypoxia as described previously.Citation29 Briefly, cells were incubated in a hypoxia chamber with oxygen concentration maintained at 1% by injecting gas mixture of 95% N2 and 5% CO2.
In vitro kinase assay
Recombinant catalytically active Nek1(Invitrogen, PV4202) and pVHL (Protein one, P2010) were incubated in kinase reaction buffer [100 mM Tris pH 7.5, 5 mM Mncl2, 2 mM DTT, 1 mM ATP and 5 µCi (γ-32P)ATP] for 20 min at 30°C. The reaction was stopped by adding 2x Laemmli buffer and boiled for 5 min at 95°C. The samples were separated on 10% Bis-Tris gels and transferred on to PVDF membranes for autoradiography. For mass spectrometry, samples were resolved on 4–12% Bis-Tris gels and stained with Commassie blue to reveal the pVHL protein band, which was exercised for mass spectrometry analysis of phosphopeptides by Taplin Mass Spectrometry Core Facility at Harvard Medical School.
Immunoprecipitation (IP)
IP was performed as described previously.Citation30 Briefly, cells were lysed in IP buffer (20 mM Tris, pH7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.15% β-maltoside, supplemented with phosphatase inhibitors and protease inhibitors) by incubating on ice for 10 min and centrifuged at 12,000 × g for 10 min to collect the supernatant lysate. About 2 ug of IP antibody was added to 500 ug of lysate and incubated overnight at 4°C on a rocker. Protein G beads were then added for overnight incubation, followed by centrifugation, three times of wash with IP buffer. The immunoprecipitate was finally collected for immunoblot analysis or for in vitro kinase assay.
Cycloheximide chase
HEK cells were transfected with VHL or its mutant. Thirty-six hours later, the cells were treated with Cycloheximide (Sigma, T7451) at 50 ug per ml to collect lysate at indicated time points for immunoblot analysis.
Immunofluorescence analysis of cilia
The method described by PlotnikovaCitation31 was followed. Briefly, cells grown on coverslips were fixed with 4% paraformaldehyde for 30 min at room temperature and washed with PBS. The fixed cells were incubated with blocking buffer (2% BSA, 0.2% fat free milk, 0.4% Triton in PBS) for 1 h, followed by incubation with the antibody against acetylated tubulin (1:10,000) for 1 h and washed with PBS containing 0.2% Tween-20. The cells were then incubated with Cy3-conjugated anti-mouse secondary antibody (Jakson Immuno Research laboratories, 715-165-150) for 1 h and washed three times. Finally, the cells were stained for nuclei with Hoechst33342 for 10 min, washed with PBS and mounted on the glass slide using antifade mounting media. To evaluate the percentage of cells with cilia, four random fields of about 35–40 cells per field were counted. Cilia length was measured using the LSM image browser software.
Additional material
Download Zip (1.2 MB)Acknowledgments
We thank Dr. William Kaelin at Harvard Medical School for providing the HA-VHL-pRc-CMV plasmid and Dr. Celeste Simon at University of Pennsylvania for RCC4 cells. The study was supported in part by research grants from the National Institutes of Health and Department of Veteran’s Affairs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol 2004; 22:4991 - 5004; http://dx.doi.org/10.1200/JCO.2004.05.061; PMID: 15611513
- Kaelin WG. Von Hippel-Lindau disease. Annu Rev Pathol 2007; 2:145 - 73; http://dx.doi.org/10.1146/annurev.pathol.2.010506.092049; PMID: 18039096
- Haase VH. The VHL tumor suppressor in development and disease: functional studies in mice by conditional gene targeting. Semin Cell Dev Biol 2005; 16:564 - 74; http://dx.doi.org/10.1016/j.semcdb.2005.03.006; PMID: 15908240
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE 2007; 2007:cm8; http://dx.doi.org/10.1126/stke.4072007cm8; PMID: 17925579
- Kaelin WG Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008; 30:393 - 402; http://dx.doi.org/10.1016/j.molcel.2008.04.009; PMID: 18498744
- Haase VH. The VHL tumor suppressor: master regulator of HIF. Curr Pharm Des 2009; 15:3895 - 903; http://dx.doi.org/10.2174/138161209789649394; PMID: 19671042
- Li M, Kim WY. Two sides to every story: the HIF-dependent and HIF-independent functions of pVHL. J Cell Mol Med 2011; 15:187 - 95; http://dx.doi.org/10.1111/j.1582-4934.2010.01238.x; PMID: 21155973
- Ohh M, Yauch RL, Lonergan KM, Whaley JM, Stemmer-Rachamimov AO, Louis DN, et al. The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol Cell 1998; 1:959 - 68; http://dx.doi.org/10.1016/S1097-2765(00)80096-9; PMID: 9651579
- Tang N, Mack F, Haase VH, Simon MC, Johnson RS. pVHL function is essential for endothelial extracellular matrix deposition. Mol Cell Biol 2006; 26:2519 - 30; http://dx.doi.org/10.1128/MCB.26.7.2519-2530.2006; PMID: 16537898
- Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat Cell Biol 2003; 5:64 - 70; http://dx.doi.org/10.1038/ncb899; PMID: 12510195
- Thoma CR, Frew IJ, Hoerner CR, Montani M, Moch H, Krek W. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat Cell Biol 2007; 9:588 - 95; http://dx.doi.org/10.1038/ncb1579; PMID: 17450132
- O’regan L, Blot J, Fry AM. Mitotic regulation by NIMA-related kinases. Cell Div 2007; 2:25; http://dx.doi.org/10.1186/1747-1028-2-25; PMID: 17727698
- White MC, Quarmby LM. The NIMA-family kinase, Nek1 affects the stability of centrosomes and ciliogenesis. BMC Cell Biol 2008; 9:29; http://dx.doi.org/10.1186/1471-2121-9-29; PMID: 18533026
- Moniz L, Dutt P, Haider N, Stambolic V. Nek family of kinases in cell cycle, checkpoint control and cancer. Cell Div 2011; 6:18; http://dx.doi.org/10.1186/1747-1028-6-18; PMID: 22040655
- Natoli TA, Gareski TC, Dackowski WR, Smith L, Bukanov NO, Russo RJ, et al. Pkd1 and Nek8 mutations affect cell-cell adhesion and cilia in cysts formed in kidney organ cultures.. Am J Physiol Renal Physiol 2008; 294:F73 - 83; http://dx.doi.org/10.1152/ajprenal.00362.2007; PMID: 17928412
- Chen Y, Chen PL, Chen CF, Jiang X, Riley DJ. Never-in-mitosis related kinase 1 functions in DNA damage response and checkpoint control. Cell Cycle 2008; 7:3194 - 201; http://dx.doi.org/10.4161/cc.7.20.6815; PMID: 18843199
- Chen Y, Chen CF, Riley DJ, Chen PL. Nek1 kinase functions in DNA damage response and checkpoint control through a pathway independent of ATM and ATR. Cell Cycle 2011; 10:655 - 63; http://dx.doi.org/10.4161/cc.10.4.14814; PMID: 21301226
- Upadhya P, Birkenmeier EH, Birkenmeier CS, Barker JE. Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc Natl Acad Sci USA 2000; 97:217 - 21; http://dx.doi.org/10.1073/pnas.97.1.217; PMID: 10618398
- Thiel C, Kessler K, Giessl A, Dimmler A, Shalev SA, von der Haar S, et al. NEK1 mutations cause short-rib polydactyly syndrome type majewski. Am J Hum Genet 2011; 88:106 - 14; http://dx.doi.org/10.1016/j.ajhg.2010.12.004; PMID: 21211617
- Shalom O, Shalva N, Altschuler Y, Motro B. The mammalian Nek1 kinase is involved in primary cilium formation. FEBS Lett 2008; 582:1465 - 70; http://dx.doi.org/10.1016/j.febslet.2008.03.036; PMID: 18387364
- Schoenfeld AR, Davidowitz EJ, Burk RD. Elongin BC complex prevents degradation of von Hippel-Lindau tumor suppressor gene products. Proc Natl Acad Sci USA 2000; 97:8507 - 12; http://dx.doi.org/10.1073/pnas.97.15.8507; PMID: 10900011
- Mohan S, Burk RD. von Hippel-Lindau protein complex is regulated by cell density. Oncogene 2003; 22:5270 - 80; http://dx.doi.org/10.1038/sj.onc.1206592; PMID: 12917628
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999; 399:271 - 5; http://dx.doi.org/10.1038/20459; PMID: 10353251
- Esteban MA, Harten SK, Tran MG, Maxwell PH. Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein. J Am Soc Nephrol 2006; 17:1801 - 6; http://dx.doi.org/10.1681/ASN.2006020181; PMID: 16775032
- Ampofo E, Kietzmann T, Zimmer A, Jakupovic M, Montenarh M, Götz C. Phosphorylation of the von Hippel-Lindau protein (VHL) by protein kinase CK2 reduces its protein stability and affects p53 and HIF-1alpha mediated transcription. Int J Biochem Cell Biol 2010; 42:1729 - 35; http://dx.doi.org/10.1016/j.biocel.2010.07.008; PMID: 20637892
- Roe JS, Kim HR, Hwang IY, Ha NC, Kim ST, Cho EJ, et al. Phosphorylation of von Hippel-Lindau protein by checkpoint kinase 2 regulates p53 transactivation. Cell Cycle 2011; 10:3920 - 8; http://dx.doi.org/10.4161/cc.10.22.18096; PMID: 22071692
- Lolkema MP, Gervais ML, Snijckers CM, Hill RP, Giles RH, Voest EE, et al. Tumor suppression by the von Hippel-Lindau protein requires phosphorylation of the acidic domain. J Biol Chem 2005; 280:22205 - 11; http://dx.doi.org/10.1074/jbc.M503220200; PMID: 15824109
- Hergovich A, Lisztwan J, Thoma CR, Wirbelauer C, Barry RE, Krek W. Priming-dependent phosphorylation and regulation of the tumor suppressor pVHL by glycogen synthase kinase 3. Mol Cell Biol 2006; 26:5784 - 96; http://dx.doi.org/10.1128/MCB.00232-06; PMID: 16847331
- Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol 2010; 176:1181 - 92; http://dx.doi.org/10.2353/ajpath.2010.090594; PMID: 20075199
- Pabla N, Ma Z, McIlhatton MA, Fishel R, Dong Z. hMSH2 recruits ATR to DNA damage sites for activation during DNA damage-induced apoptosis. J Biol Chem 2011; 286:10411 - 8; http://dx.doi.org/10.1074/jbc.M110.210989; PMID: 21285353
- Plotnikova OV, Pugacheva EN, Golemis EA. Primary cilia and the cell cycle. Methods Cell Biol 2009; 94:137 - 60; http://dx.doi.org/10.1016/S0091-679X(08)94007-3; PMID: 20362089