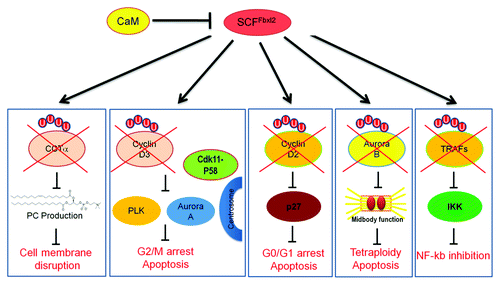
Ubiquitin E3 ligases represent an increasingly diverse group of proteins whose precise biologic role still remains enigmatic. Of the SCF (Skp1-Cullin1-F-box) E3 ligase family, for example, only very few subunits from over 60 family members are well-characterized. The SCF apparatus contains a multi-subunit catalytic core consisting of Skp1, Cullin1 and the E2 ubiquitin-conjugating (Ubc) enzyme and an F box receptor-like component that targets many substrates. The F-box proteins have two functional domains: an NH2-terminal F-box motif that binds Skp1, and a C-terminal leucine-rich repeat (LRR), WD motif or another signature that recognizes substrates.Citation1 SCF ligase complexes are critically involved in regulating DNA repair, cell cycle progression, inflammation, centrosome stability, mitotic fidelity and cellular proliferation.Citation2 Hence, it is not surprising that novel targeted therapies might involve modulating activities of SCF subunits in cells during neoplasia, cell growth and repair or inflammation.
Following its initial description, F-box protein Fbxl2 was shown to interact with hepatitis C virus.Citation3 However, until recently, the authentication of Fbxl2 as a ubiquitin E3 ligase component and its molecular behavior was not demonstrated. Recent studies show that Fbxl2 acts as the receptor component of a classical SCF ubiquitin E3 ligase, recognizing a calmodulin (CaM)-binding signature.Citation4 CaM is a highly conserved protein that binds to its targets in a calcium-dependent or calcium-independent manner by recognizing specific molecular signatures including an IQ motif (I/LQXXXRGXXXR), a 1-10 or 1-5-10-binding motif.Citation5
Indeed, recognition of an IQ motif by Fbxl2 represents an unusual characteristic of F box proteins that typically are recruited by phosphodegrons within target substrates.
The first authenticated substrate of Fbxl2 is CTP: phosphocholine cytidylyltransferase (CCTα), a key rate-regulatory enzyme that is indispensable for membrane phosphatidylcholine production. Fbxl2 is sufficient to mediate CCTα monoubiquitination in a calcium-dependent process through actions of the SCFFbxl2 complex. However, both CaM and Fbxl2 competitively interact to vie for occupancy within a canonical IQ motif within CCTα, where CaM acts as a stabilizing ligand for CCTα protein. This competition model reveals that CaM acts as an antagonist of the SCF subunit to oppose Fbxl2-mediated substrate ubiquitination.Citation4
Fbxl2 also targets cell cycle proteins. The SCFFbxl2 complex targets cyclin D2 in B-lymphocytes and leukemic cells, resulting in G0 arrest and apoptosis.Citation6 Cyclin D2 is the dominant D-type cyclin with leukemic cells and is essential for G0/G1 phase progression, which translocates the cyclin-dependent kinase inhibitor, p27, out of the nucleus. Fbxl2 also ubiquitinates cyclin D3 and impairs G2/M transition.Citation7 Cyclin D3 is a multi-functional protein that directly interacts with and confers activity for CDK11p58 that controls centrosome maturation and bipolar spindle formation.Citation8
Both cyclin D2 and cyclin D3 harbor canonical IQ motifs typical of calcium-independent CaM binding proteins. Within the IQ motif, it appears that the glutamine residue is essential for Fbxl2 targeting as cyclin D2 and cyclin D3 variants harboring glutamine substitutions within the IQ motif exhibit significantly extended half-lives in vivo. Interestingly, threonine286, within cyclin D1, is recognized by the F-box proteins Fbxo4, Fbxw8 and Fbxo31 to facilitate its ubiquitination.Citation9 Although this threonine site is highly conserved among the D cyclins, Fbxl2 did not utilize this molecular signal for targeting, but rather docks within a consensus IQ signature within these cyclins to facilitate their ubiquitination.
The intermolecular competition between SCFFbxl2 complex and CaM regulate other proteins involved in mitotic progression. Recently, we identified that CaM, acting as a sensor of chromosome bridges, protects Aurora B at the midbody to stabilize the ingressed furrow for delayed abscission (unpublished data). However, these activities are opposed by Fbxl2, which again engages CaM for access to Aurora B within a shared IQ motif. Last, chemical inhibition of CaM was highly effective in reducing both Aurora B levels and tumor viability. These observations implicate a role for the F-box protein and CaM as diametric homeostatic sensors that regulate the cytokinesis program through modulation of Aurora B concentrations.
More recent studies reveal that the SCFFbxl2 complex might also play an important role in regulating the NFκB pathway and cytokine responses. Fbxl2 acts as a crucial, pan-reactive inhibitor of tumor necrosis factor receptor-associated factors (TRAF) function by mediating their polyubiquitination.Citation10 By eliminating TRAFs, Fbxl2 inhibits NFκB activity and secretion of a broad spectrum of Th1 panel cytokines. Interestingly, TRAF proteins contain a conserved 1–10 CaM-binding motif in which a tryptophan residue is essential for Fbxl2 targeting.Citation10 In conclusion, studies in our laboratory have identified multiple substrates of Fbxl2, all of which are CaM-binding proteins and are essential for either cell proliferative activity or innate immunity. The ability of Fbxl2 to recognize a CaM binding signature appears to represent a unique molecular mechanism of F box protein substrate targeting that underlies its ability to exhibit tumorocidal activity and possibly play a role in immune suppression. Indeed, Fbxl2 expression is significantly reduced in peripheral blood cells from leukemic subjects, where its substrates cyclin D3, cyclin D2, Aurora B are highly expressed.Citation6 Additional investigations are needed to assess the expression of Fbxl2 in subjects with inflammatory illness, such as sepsis and pneumonia. ()
References
- Cenciarelli C, et al. Curr Biol 1999; 9:1177 - 9; http://dx.doi.org/10.1016/S0960-9822(00)80020-2; PMID: 10531035
- Skaar JR, et al. Cell 2009; 137:1160 - e1, e1; http://dx.doi.org/10.1016/j.cell.2009.05.039; PMID: 19524517
- Wang C, et al. Mol Cell 2005; 18:425 - 34; http://dx.doi.org/10.1016/j.molcel.2005.04.004; PMID: 15893726
- Chen BB, et al. Mol Cell Biol 2011; 31:1905 - 20; http://dx.doi.org/10.1128/MCB.00723-10; PMID: 21343341
- Rhoads AR, et al. FASEB J 1997; 11:331 - 40; PMID: 9141499
- Chen BB, et al. Blood 2012; 119:3132 - 41; http://dx.doi.org/10.1182/blood-2011-06-358911; PMID: 22323446
- Chen BB, et al. Oncogene 2012; 31:2566 - 79; http://dx.doi.org/10.1038/onc.2011.432; PMID: 22020328
- Chen BB, et al. Cell Cycle 2011; 10:3487 - 94; http://dx.doi.org/10.4161/cc.10.20.17742; PMID: 22024926
- Santra MK, et al. Nature 2009; 459:722 - 5; http://dx.doi.org/10.1038/nature08011; PMID: 19412162
- Chen BB, et al. Nat Immunol 2013; In press