Keywords: :
The self-renewal capacity of stem cells is crucial for the homeostatic maintenance of adult tissues, in which they mediate the continuous replacement of differentiated cells, and is the focus of attempts to design patient-specific therapies.Citation1 The Polycomb group proteins are key global epigenetic regulators of stem-cell fate decisions. The Polycomb family induces histone-specific posttranslational modifications on target genes through the action of the two Polycomb repressive complexes (PRC1 and PRC2). PRC2 catalyzes methylation of Lys27 on histone 3 (H3), generating the H3K27me2/3 mark, which acts as a platform for PRC1, which, in turn, ubiquitylates histone H2A on Lys119.Citation2 The catalytic subunits of the PRC1 and PRC2 complexes are, respectively, Ring1B and Ezh1/2, but it should be noted that the repressive activity of the Polycomb group also requires the presence of other functional subunits.Citation3
The chromatin-modifying component of PRC2 exists in two forms, Ezh1 and Ezh2, which have an identical catalytic SET domain. Therefore the exclusive attribution of methyltransferase activity to Ezh2 needs to be retested; indeed, accumulated evidence support the hypothesis that the repressive activities of PRC2-Ezh1 and PRC2-Ezh2 are differentially coordinated during tissue development and homeostasis. The H3K27me3 mark is not completely lost in Ezh2−/− ESCs, suggesting residual or compensatory methylation by Ezh1.Citation4 Moreover, Ezh1 is indispensable for the establishment of pluripontent cells and for the maintenance of ESC self-renewal.Citation5 Further evidence for a role for Ezh1 comes from the relatively mild phenotypes of mice after conditional targeting of Ezh2 in somatic stem cells and the complementary spatial and temporal expression patterns of Ezh1 and Ezh2 during development.Citation5 It is therefore important to determine the role of Ezh1-containing PRC2 complexes in the maintenance of adult stem cells. Our recent findings show that Ezh1 is important for physiological HSC activation.Citation6 We propose that Ezh1 might balance HSC preservation against the risk of aging, since disturbance of PRC2-Ezh1 function affects several important cell fate decisions, including senescence and self-renewal.
The extreme sensitivity of HSCs to Ezh1 inactivation in mice is remarkable when compared with the impact of deficiency for Ezh2 in a similar system.Citation5 Ezh1 deficiency induces a potent senescence response that severely reduces the size of the adult HSC fraction as well as the self-renewal capacity and quiescent population of HSCs. This specialized function is related to specific regulation by Ezh1 of mono- and dimethylation of H3K27, which prevents active proliferation and represses pathways required for terminal differentiation and senescence of adult HSCs.
The challenge of revealing Ezh1-mediated mechanisms was met by genome-wide analyses combined with quantification of ChIP assays in HSCs rendered senescent by Ezh1 deletion. We identified a role for Ezh1 in coordinated methylation of the repressive H3K27 mark, resulting in activation of the master senescence regulators p16INK4a and Bmp2Citation7 and selective repression of Runx1, a transcription factor that regulates critical processes in many aspects of hematopoiesis.Citation8 Repression of Runx1 is achieved by dynamic and harmonized H3K27me1 and H3K27me2 alterations, but not upon trimethylation, to generate the H3K27me3 mark. Thus the unique and specialized function of Ezh1, mediated through mono- and di-methylation of H3K27, is to prevent active proliferation and repress pathways required for terminal senescence. Based on these findings, we propose a model in which the energy needed to recruit PRC2 to target gene promoters is the sum of the energy steps established by each of the PRC2 holoenyzyme components (). We suggest that H3K27me1 is an important intermediary PCR2-Ezh1 product, because it not only constitutes the substrate for subsequent H3K27me2, but also prevents H3K27 from being acetylated. Despite the variety of proteins associated with the core PCR2 complex, its integrity should remain intact, such that all PRC2 complexes containing either Ezh1 or Ezh2 catalyze H3K27 methylation.
Figure 1. In the hematopoietic system, where stem cell activation is a transitory state that requires a combination of self-renewal coupled with the prevention of differentiation and senescence, Ezh1 is needed to maintain primitive hematopoietic cells by protecting them from differentiation and senescence in slow cycling through its PRC2-associated Polycomb function.
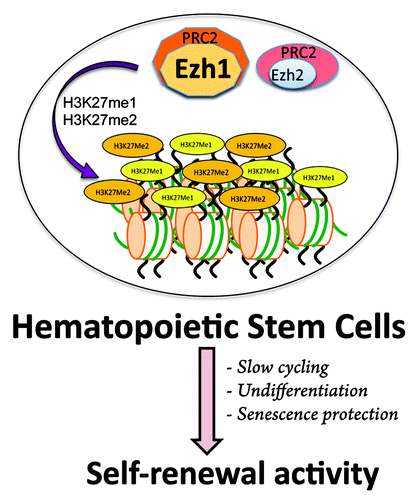
Our knowledge of Ezh1 has lagged behind that of other epigenetic regulators. Now, the identification of PRC2-Ezh1 as responsible for H3K27me1 and H3K27me2 marks in HSCs opens the way to exciting discoveries about the biological function of these epigenetic marks. A combination of mechanistic and functional studies will be required to determine whether and how misregulation of histone methyltransferases Ezh1 and Ezh2 affects specific modes of chromatin recruitment, and how these enzymes exert distinct biological functions.
References
- Muller-Sieburg C, Sieburg HB. Stem cell aging: survival of the laziest?. Cell Cycle 2008; 7:3798 - 804; http://dx.doi.org/10.4161/cc.7.24.7214; PMID: 19066464
- Bracken AP, Helin K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nat Rev Cancer 2009; 9:773 - 84; http://dx.doi.org/10.1038/nrc2736; PMID: 19851313
- Luis NM, Morey L, Di Croce L, Benitah SA. Polycomb in stem cells: PRC1 branches out. Cell Stem Cell 2012; 11:16 - 21; http://dx.doi.org/10.1016/j.stem.2012.06.005; PMID: 22770239
- Surface LE, Thornton SR, Boyer LA. Polycomb group proteins set the stage for early lineage commitment. Cell Stem Cell 2010; 7:288 - 98; http://dx.doi.org/10.1016/j.stem.2010.08.004; PMID: 20804966
- Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature 2011; 469:343 - 9; http://dx.doi.org/10.1038/nature09784; PMID: 21248841
- Hidalgo I, Herrera-Merchan A, Ligos JM, Carramolinos L, Dominguez O, Torres M, et al. Ezh1 is required for hematopoietic stem cell function by preventing senescence-like cell cycle arrest. Cell Stem Cell 2012; 11:1 - 14; http://dx.doi.org/10.1016/j.stem.2012.08.001; PMID: 22770234
- Kaneda A, Fujita T, Anai M, Yamamoto S, Nagae G, Morikawa M, et al. Activation of Bmp2-Smad1 signal and its regulation by coordinated alteration of H3K27 trimethylation in Ras-induced senescence. PLoS Genet 2011; 7:e1002359; http://dx.doi.org/10.1371/journal.pgen.1002359; PMID: 22072987
- Swiers G, de Bruijn M, Speck NA. Hematopoietic stem cell emergence in the conceptus and the role of Runx1. Int J Dev Biol 2010; 54:1151 - 63; http://dx.doi.org/10.1387/ijdb.103106gs; PMID: 20711992