Polymorphonuclear neutrophil granulocytes (neutrophils) represent a fundamental component of the innate immune system, forming an efficient barrier against invading microbial pathogens. Neutropenia, a common unwanted side effect of chemotherapy, is associated with infectious complications. The human bone marrow produces an estimated number of 1011 neutrophils per day, and granulopoiesis physiologically takes up two-thirds of the hematopoietic marrow space.
The regulation of neutrophil numbers in the steady state remains incompletely understood. More recent evidence, however, suggests that it depends on separate hematopoietic signaling pathways as compared with “emergency granulopoiesis,” elicited by injection of infectious agents into experimental mice.Citation1 A “neutrostat” has been suggested to detect neutrophils released by the marrow (“turnstile” hypothesis) or, alternatively, to measure the number of apoptotic neutrophils phagocytosed by tissue macrophages.Citation2
Indeed, adhesion molecule-deficient mice, whose neutrophils are unable to transmigrate, display pronounced neutrophilia. The underlying mechanism was elegantly shown to represent disinhibited macrophage-derived IL-23 secretion, which, in turn, stimulates TH17-derived IL-17 production. IL-17 boosts G-CSF secretion, which stimulates granulopoiesis.Citation3 Further evidence derived from experiments in macrophage and dendritic cell-deficiency models confirm that phagocytes are able to generate a negative feedback signal for granulopoiesis (reviewed in ref. Citation2). However, marrow-derived macrophages, but not peritoneal macrophages, secrete G-CSF in a dose-dependent manner upon phagocytosis of apoptotic neutrophils.Citation4 In summary, there is experimental data for both positive and negative regulatory effects of macrophages on steady-state granulopoiesis.
A putative neutrostat should ideally sense functional shortages in total neutrophil mass rather than merely quantify ongoing production or consumption. Treatment of experimental mice with anti-neutrophil antibodies effectively depletes neutrophils. Feedback responses include phenomena indistinguishable from a status post application of G-CSF, namely increased neutrophil production and mobilization from the marrow. This robust feedback mechanism has recently been shown to be independent of lymphocytes or NK cells (NOD/SCIDcγ−/− mice), independent of the inflammasome (IL1R/IL1β), independent of the emergency granulopoiesis regulator CEBPβ, but dependent on G-CSF.Citation5,Citation6
G-CSF is the major cytokine regulating granulopoiesis, and G-CSF−/− or G-CSF-R−/− mice display reduced neutrophil numbers (20–30% of normal) in the steady state. However, emergency granulopoiesis can be induced even in G-CSF and GM-CSF doubly deficient mice. The fact that low-level granulopoiesis is preserved in animals with absent G-CSF signaling indicates redundant regulatory mechanisms not only in emergency situations, but also in the steady state. Candidate signaling pathways include receptors of pathogen-associated molecular patterns (PAMPs) provided by commensal flora, which modulates both innate and adaptive immunity.
Indeed, functional maturation of granulocytes was demonstrated to be dependent on microbiota-derived ligands of the pattern recognition receptor (PRR) NOD1.Citation7 Moreover, germ-free kept (GF) wild-type mice exhibit even more pronounced neutropenia than G-CSF-deficient animals. Further enhancement of neutropenia in GF mice by neutrophil depleting antibodies induces feedback granulopoiesis similar to control mice, including significantly increased G-CSF levels.Citation5 In summary, study of germ-free mice reveals low-level granulopoiesis with intact feedback loops upon neutropenia. However, analyses of neutropenic TLR4−/− and TRIF−/− mice revealed a dysfunctional regulatory feedback loop including upregulation of G-CSF, while it was preserved in MyD88−/− and TLR2−/− animals. In summary, PRR signaling via TLR4 and its adaptor protein TRIF is indispensable in steady-state granulopoiesis. The TLR4-expressing sensor is sensitive to such an extent that even minute amounts of PAMPs ingested with autoclaved food may be sufficient to convey the neutropenia signal in GF mice. Alternatively, endogenous TLR4 agonists active upon neutrophil depletion may play a role in the GF model.
In conclusion, the same highly conserved regulatory mechanisms are responsible for both steady-state and emergency granulopoiesis, and the prevailing apodictic separation has to be given up. Current evidence suggests that granulopoiesis represents a demand-driven process triggered along a continuum, likely by exposure to PAMPs and thereby reflecting the mutual relationship of microbiota and host immunity.
Two major questions remain unanswered:
(1) Which TLR4-positive cellular compartment represents the neutrostat?
The putative neutrostat in the model of induced neutropenia is dependent on TLR4. In a model of LPS-induced emergency granulopoiesis, the sensing cellular elements were not transplantable with whole bone marrow transplantation.Citation8 Deregulated bone marrow stromal cells deficient in RARγ or dicer are sufficient to induce hyperproliferation of myelopoiesis (reviewed in ref. Citation2), and mesenchymal stromal cells express large amounts G-CSF after TLR4-dependent LPS stimulation.Citation5 However, candidates not only include non-hematopoietic (stromal) marrow components such as MSCs or endothelial cells, but also cellular compartments outside the marrow.
(2) Which exogenous or endogenous TLR4 ligands are involved in steady-state granulopoiesis?
LPS has been shown to directly signal into the marrow hematopoietic stem and progenitor cell (HSPC) niche and influence the divisional and differential fate of HSC. However, endogenous molecules also activate and/or coactivate TLRs. It is therefore conceivable that very low-level PAMPs may convey signals via endogenously sensitized or modulated TLR4.
Further research answering these questions is necessary to completely unravel the phenomenon of steady-state granulopoiesis. A better understanding will help us to treat patients in neutropenia—both associated with chemotherapy and idiopathic. ()
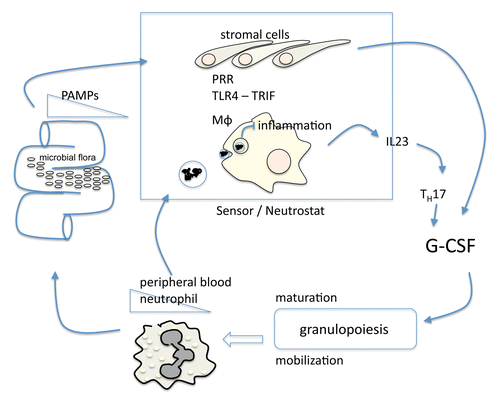
Figure 1. Schematic overview over steady-state granulopoiesis. PAMPs (pathogen-associated molecular patterns) may originate from the dense intestinal microflora and bind to PRRs (pattern recognition receptors) expressed by both hematopoietic and non-hematopoietic TLR4+ sensing cells forming the neutrostat. Downstream signaling pathways culminate in G-CSF dependent granulopoiesis. Mϕ, macrophage.
References
- Hirai H, et al. Nat Immunol 2006; 7:732 - 9; http://dx.doi.org/10.1038/ni1354; PMID: 16751774
- Bugl S, et al. Academy of. Sciences (New York) 2012; 1266:171 - 8; http://dx.doi.org/10.1111/j.1749-6632.2012.06607.x
- Stark MA, et al. Immunity 2005; 22:285 - 94; http://dx.doi.org/10.1016/j.immuni.2005.01.011; PMID: 15780986
- Furze RC, et al. FASEB J 2008; 22:3111 - 9; http://dx.doi.org/10.1096/fj.08-109876; PMID: 18509199
- Bugl S, et al. Blood 2013; 121:723 - 33; http://dx.doi.org/10.1182/blood-2012-05-429589; PMID: 23223360
- Cain DW, et al. PLoS ONE 2011; 6:e19957; http://dx.doi.org/10.1371/journal.pone.0019957; PMID: 21655273
- Clarke TB, et al. Nat Med 2010; 16:228 - 31; http://dx.doi.org/10.1038/nm.2087; PMID: 20081863
- Boettcher S, et al. J Immunol 2012; 188:5824 - 8; http://dx.doi.org/10.4049/jimmunol.1103253; PMID: 22586037