Abstract
The amount of pericentriolar matrix at the centrosome is tightly linked to both microtubule nucleation and centriole duplication, although the exact mechanism by which pericentriolar matrix levels are regulated is unclear. Here we show that Centrobin, a centrosomal protein, is involved in regulating these levels. Interphase microtubule arrays in Centrobin-depleted cells are more focused around the centrosome and are less stable than the arrays in control cells. Centrobin-depleted cells initiate microtubule nucleation more rapidly than control cells and exhibit an increase in the number of growing microtubule ends emanating from the centrosome, while the parameters of microtubule plus end dynamics around the centrosome are not significantly altered. Finally, we show that Centrobin depletion results in the increased recruitment of pericentriolar matrix proteins to the centrosome, including γ-tubulin, AKAP450, Kendrin and PCM-1. We propose that Centrobin might regulate microtubule nucleation and organization by controlling the amount of pericentriolar matrix.
Introduction
The centrosome is critical for the nucleation and organization of the microtubule network in many cell types, where it functions to regulate cell shape, migration and cell cycle progression. Consisting of a pair of centrioles surrounded by an electron-dense cloud of pericentriolar matrix (PCM), the centrosome duplicates once per cell cycle when a daughter centriole assembles next to the pre-existing mother centriole.
A number of proteins required for centriole duplication including Plk4, hSAS-6 and CPAP are sequentially recruited to the centrioles.Citation1 While these proteins are conserved among higher eukaryotes, the process of centriole assembly is quite divergent between species.Citation2 Despite this, a number of studies in different eukaryotic models have shown a relationship between PCM recruitment and centriole biogenesis. For example, in C. elegans depletion of centriole-duplicating factor ZYG-1 (Plk4 ortholog) results in a decrease in PCM recruitment corresponding with a reduction in microtubule nucleation.Citation3 While comparable studies are lacking in humans, disruption of the centrioles has been shown to result in PCM dispersion.Citation4 The mechanism for this is unclear, although it has been suggested that the presence of centrosome duplication factors endows the centriole with the ability to recruit PCM.Citation3
Centrobin is a centrosomal protein that was initially described as a centriole-duplication factor.Citation5 More recently, Centrobin has been shown to have microtubule-bundling activity,Citation6 and it has been proposed that it might stabilize microtubules during mitosis by anchoring the centrosome to the mitotic spindle.Citation6,Citation7 The role of Centrobin in organizing interphase microtubules is not well defined, although it has been previously shown that its depletion results in disorganization of the microtubule network. Here, we show that the microtubules become more focused around the centrosome in Centrobin-depleted interphase cells, concomitant with an increase in microtubule nucleation and defects in microtubule stability. Centrobin-depleted cells also exhibited an increase in PCM recruitment to the centrosome. We propose that the defects in microtubule organization and nucleation observed in Centrobin-depleted cells are due to its role in limiting PCM recruitment.
Results
Centrobin regulates interphase microtubule organization and stability
Centrobin has previously been shown to play a role in the regulation of mitotic spindle dynamics,Citation6,Citation7 but its role in regulation of interphase microtubule dynamics has not been determined. To explore this, we made a bacterial artificial chromosome encoding GFP-Centrobin and stably expressed it in HeLa cells at near-endogenous levels to determine if Centrobin also localizes to the microtubules in interphase cells. Staining of the GFP-Centrobin with an anti-GFP antibody revealed that it localizes exclusively to the centrosome during interphase, as determined by colocalization with γ-tubulin (). Next, we examined the organization of microtubules in control and Centrobin-depleted cells by staining cells with antibodies against α-tubulin. Microtubule arrays in Centrobin-depleted cells appeared more focused around the centrosome, whereas the control cells displayed a more even distribution of microtubules (). In particular, the fluorescence intensity of α-tubulin near the cell edge was approximately 30% less than control cells, suggesting that there are fewer microtubules extending all the way to the cell periphery. By contrast, no defects were observed in either the actin fibers or intermediate filaments (Fig. S1).
Figure 1. Centrobin-depleted cells exhibit a redistribution of the microtubule network and microtubule stability defect. (A) HeLa cells stably expressing GFP-Centrobin at near-endogenous levels were fixed and co-stained for GFP, γ-tubulin and DAPI. Scale bar represents 10 μm. (B–D) Control or Centrobin-depleted (CENTsi) HeLa cells were harvested for (B) western blotting with the indicated antibodies or (C) co-stained for α-tubulin, Kendrin and DAPI. Scale bar represents 20 μm. (D) The intensity of microtubule staining from (C) was measured at the densest region of microtubules near the cell center, at the cell edge closest to this region and midway between the two points by quantifying the intensity of a 2.14 μm square at each point. For each experiment, 50 cells were measured per condition. Error bars represent the standard error of the mean from three independent experiments. p-values are denoted as follows: *p < 0.05.
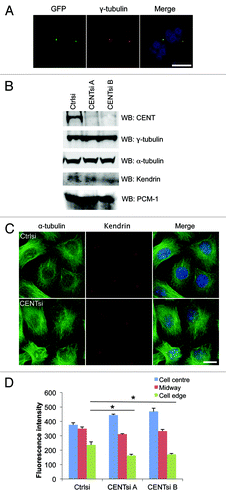
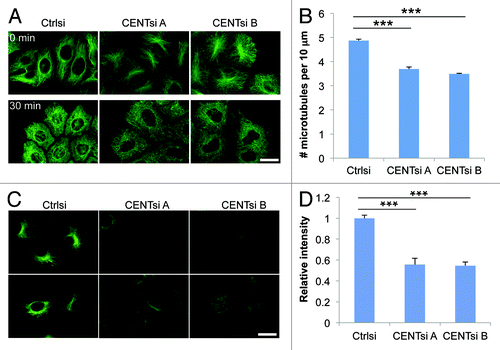
To determine if changes in microtubule organization were accompanied by altered microtubule stability, we analyzed nocodazole-induced microtubule depolymerization in control and Centrobin-depleted cells. Microtubules in Centrobin-depleted cells disassembled somewhat more rapidly than in control cells when treated with 10 μm nocodazole for 30 min, with an average of 4.9 microtubules per 10 μm in control cells compared with 3.7 and 3.5 microtubules per 10 μm, respectively, in Centrobin-depleted cells (). To further evaluate the link between Centrobin function and microtubule stability, we examined the distribution pattern of tubulin acetylation, a post-translational tubulin modification that serves as a marker of stable microtubules,Citation8 in control and Centrobin-depleted cells. Consistent with the above results, Centrobin-depleted cells displayed approximately half the acetylated tubulin intensity of control cells (), further indicating that interphase microtubules in these cells are less stable than in control cells.
Figure 2. Centrobin-depleted cells exhibit a microtubule stability defect. (A) Control and Centrobin-depleted cells were treated with 10 μM nocodazole for 30 min and stained for α-tubulin. (B) Quantification of the average number of microtubules remaining per 10 μm after nocodazole treatment from (A). For each cell, a 10 μm line was drawn in five different positions around the cell parallel to the nearest cell membrane and the number of microtubules crossing this line counted. Twenty cells were measured for each condition, resulting in a total of 100 measurements per condition. (C) Control or Centrobin-depleted HeLa cells were stained for acetylated tubulin. (D) Quantification of relative acetylated tubulin intensity per cell from (C). For each experiment, 20 cells were measured per condition. Scale bars represent 20 μm. Error bars represent the standard error of the mean from three independent experiments. p-values are denoted as follows: ***p < 0.001.
Centrobin suppresses microtubule nucleation
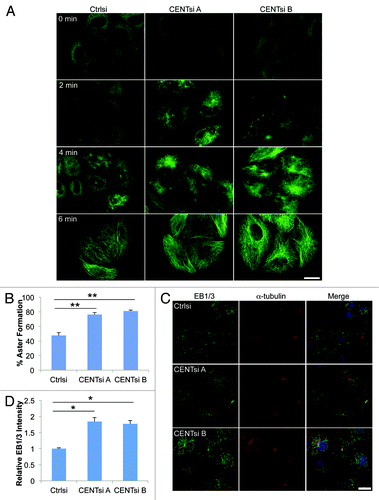
To ascertain if the more perinuclear microtubule array observed in Centrobin-depleted cells was due to altered microtubule nucleation from the centrosome, we explored the role of Centrobin in microtubule nucleation by assessing the dynamics of microtubule re-growth after depolymerization. Microtubules were depolymerized by cold treatment, and the nucleation was observed at various time points by staining for α-tubulin. We found that in Centrobin-depleted cells, microtubule re-growth was initiated more rapidly, as evidenced by an increase in the number of cells with microtubule asters after 2 min re-growth (). To explore this further, we stained cells that had been subjected to microtubule re-growth for 2 min with an antibody that detects the plus-end tracking proteins EB1 and EB3 (EB1/3). EB1/3 comets were more numerous and more focused around the centrosome in Centrobin-depleted cells compared with the control cells (), likely due to an increase in the number of short microtubules emanating from the centrosome. Furthermore, the fluorescence intensity of the EB1/3 staining was 1.8-fold higher in Centrobin-depleted cells than control cells (). A similar microtubule re-growth phenotype was seen after depolymerization with nocodazole (data not shown).
Figure 3. Centrobin-depleted cells initiate microtubule nucleation more rapidly than control cells. HeLa cells were transfected with control or Centrobin siRNA, incubated for 48 h and placed on ice for 1 h. (A) Cells were returned to warm (37°C) media for the indicated times, fixed and stained for α-tubulin. (B–D) Cells were returned to warm media for 2 min and then fixed. (B) Cells were stained for α-tubulin and quantified for the percentage of cells with aster formation after 2 min. Asters were identified by using the thresholding tool in ImageJ. For each experiment, 100 cells were counted. (C) Cells were fixed and stained for α-tubulin, EB1/3 and DAPI. (D) Quantification of total EB1/3 intensity per cell from (C) relative to control cells. For each experiment, 50 cells were counted. Error bars represent the standard error of the mean from three independent experiments. p-values are denoted as follows: *p < 0.05, **p < 0.01. Scale bars represent 20 μm.
To further investigate the role of Centrobin in regulation of microtubule dynamics, control and Centrobin-depleted cells were transfected with EB3-GFP and imaged by spinning disc confocal microscopy. Consistent with our observations in fixed cells there was a 2-fold increase in the number of EB3 comets arising from centrosomes in Centrobin-depleted cells (; Movies 1–3) but no difference was observed in comet velocity in the pericentrosomal area (Table S1), suggesting that the microtubule growth rate does not significantly change.
Figure 4. Centrobin-depleted cells exhibit increased microtubule nucleation. (A) HeLa cells were co-transfected with EB3-GFP and control or Centrobin siRNA. Maximum intensity projections of live cell image sequences of control and Centrobin-depleted cells. Images were collected every 0.5 sec, and the movies were 2 min in duration. Scale bar represents 10 μm. (B) Microtubule nucleation frequency from the centrosome as quantified from movies of EB3-GFP described in (A). A circle measuring 5 μm in diameter was drawn around the centrosome and comets originating in the circle were counted. (C) Control and Centrobin-depleted cells were co-stained with antibodies against Cyclin B1 and EB1/3. Scale bar represents 20 μm. (D) Quantification of the number of EB1/3 comets around the centrosome from fixed cells described in (C). The location of the centrosome was identified via 3D reconstruction of the cells then a maximum projection image generated and a circle measuring 5 μm in diameter was drawn around the centrosome. All the comets contained within the circle were counted. (E) Cell cycle profiles of control and Centrobin-depleted cells. Error bars represent the standard error of the mean from three independent experiments. For each of these experiments, 10 cells were measured per condition. p-values are denoted as follows: ***p < 0.001.
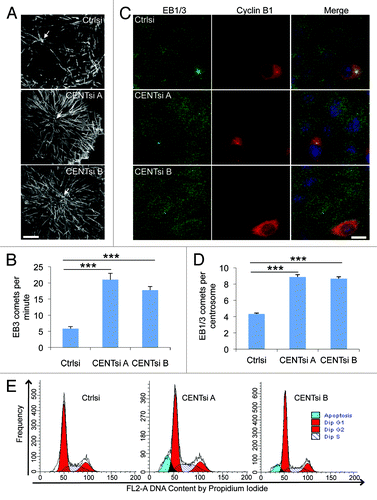
One possible explanation for the increased nucleation in Centrobin-depleted cells was that we were observing an indirect effect due to the presence of higher proportion of G2 cells. To explore this further, we co-stained cells for Cyclin B1 and EB1/3, and then counted the number of EB1 comets arising only from cells in the G1/S phase of the cell cycle (Cyclin B1-negative cells). These cells also showed a 2-fold increase in comets emanating from the centrosome (as determined by 3D reconstruction) after Centrobin-depletion (). We also analyzed the proportion of cells in each phase of the cell cycle by FACS analysis. Apart from an increase in sub-G1 cells in Centrobin-depleted cells, there was no dramatic difference in cell cycle profiles between control and Centrobin-depleted cells, further indicating that the above effects were not attributable to an alteration in the cell cycle progression of the Centrobin-depleted cells ().
To further analyze microtubule dynamics of Centrobin-depleted cells, we co-transfected cells with mCherry-α-tubulin and either control or Centrobin shRNAs and imaged the cells by total internal reflection fluorescence (TIRF) microscopy. Given the density of microtubules around the centrosome in Centrobin-depleted cells, we photobleached an area around the centrosome in each cell and observed growth of freshly polymerised microtubules in this area. Consistent with our observations of EB3-GFP, there was no significant difference in the velocity of microtubule growth, and the differences in the velocity of microtubule depolymerization were also minor (Table S1). Similarly, there were no significant differences in catastrophe or rescue frequencies, indicating that except for microtubule nucleation, the parameters of microtubule plus end dynamics in the pericentrosomal area were not significantly changed.
Centrobin limits PCM recruitment
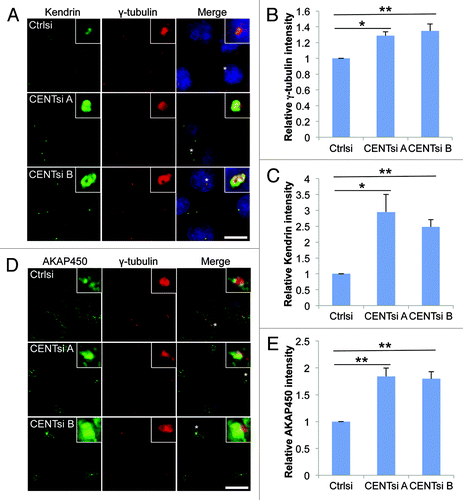
To explain how Centrobin might regulate both centriole duplication and microtubule nucleation/organization, we hypothesized that centrosome integrity might be affected by loss of Centrobin. To examine this, we screened control and Centrobin-depleted cells with a panel of centrosomal antibodies and looked for changes in localization and intensity of staining. While none of these proteins were mislocalized in Centrobin-depleted cells, we observed an increase in the intensity of PCM proteins including γ-tubulin, AKAP450, Kendrin and PCM-1 at the centrosomes (; Fig. S2A and B). In contrast, no change in intensity was observed for Centrin staining (Fig. S2C and D). The increased intensity was not due to an increase in levels of these proteins (), suggesting that Centrobin depletion results in increased PCM recruitment to the centrosome.
Figure 5. PCM accumulation at the centrosome is increased in Centrobin-depleted cells. Control or Centrobin-depleted cells were stained with the indicated antibodies: (A–C) γ-tubulin and Kendrin, (D and E) γ-tubulin and AKAP450. Insets are 8× magnification of centrosomes marked with asterisk. Intensity of the centrosomal staining was measured by drawing a 2.14 μm square around the centrosome and is graphed relative to control cells. Error bars represent the standard error of the mean from three independent experiments. For each experiment 20 cells were counted per condition. p-values are denoted as follows: *p < 0.05, **p < 0.01. Scale bars represent 20 μm.
Discussion
Microtubules nucleate from γ-tubulin ring complexes, which reside in the PCM, anchored to the distal and sub-distal appendages of centrioles.Citation9 While several proteins are known to be required for microtubule nucleation,Citation10,Citation11 our results provide evidence that Centrobin acts as a distinct type of regulator: it suppresses microtubule nucleation in interphase cells. This view came from microtubule repolymerization assays, where Centrobin-depleted cells initiated microtubule regrowth more rapidly than the control cells. Live cell imaging of Centrobin-depleted cells expressing EB3-GFP then revealed that centrosomes in Centrobin-depleted cells initiate twice as many EB3 comets per minute as control cells. Furthermore, these changes were observed in fixed G1/S (Cyclin B1-negative cells), indicating that this effect was not due to a change in cell cycle distribution.
Theoretical work on microtubule dynamicsCitation12-Citation15 suggested that an increase in microtubule nucleation would cause a decrease in the soluble tubulin pool, because more of the tubulin in the cell would be shifted into the polymer form. This effect would indirectly affect microtubule plus-end dynamics, for example, by causing a decrease in microtubule polymerization rate. However, the parameters of microtubule growth and shortening at the plus end remained unchanged in Centrobin-depleted cells. An interesting possibility is that an increase in centrosomal nucleation was accompanied by an increased detachment of microtubule minus ends from the centrosome. In this way, microtubule depolymerization from the minus ends would replenish the tubulin pool, explaining why microtubule plus end dynamics was not affected. If this view were correct, the overall stability and longevity of microtubules would decrease, which fits well with the observed reduction of microtubule acetylation, a marker of stable microtubules, after Centrobin knockdown.
Centrioles duplicate once per cell cycle and require a number of highly conserved centriole-duplication factors, including Plk4 and SAS6.Citation1 Despite this, differences in the duplication process have been noted between worms, flies and humans.Citation2 With increased levels of complexity in higher eukaryotes, additional layers of regulation are required. Centrobin is only expressed in higher eukaryotes and is known to control centriole duplication by promoting the elongation and stability of centrioles.Citation16
It has been suggested that the accumulation of centriole-duplication factors at the centrosome allows PCM proteins to bind.Citation3 As such, depletion of a centriole-duplication factor such as Centrobin might be expected to result in a reduction of the PCM at the centrosome. However, we observed an increase in PCM at the centrosome. We propose that Centrobin negatively regulates the microenvironment of the centrosome to limit PCM recruitment. Thus, Centrobin might play a role in regulating both centriole duplication and PCM recruitment, as has been described for a number of other centrosomal proteins.Citation3
The mechanism by which this may occur is unclear. However, it has recently been shown in Drosophila that the GTP state of tubulin plays a role in PCM recruitment.Citation17 Specifically, tubulin-GTP prevents SAS-4 (CPAP ortholog) from forming complexes with other PCM proteins, whereas tubulin-GDP promotes it. Centrobin has previously been shown to interact with tubulin, but the effect of GTP hydrolysis on the interaction was not examined.Citation16 Perhaps the ability of Centrobin to limit PCM recruitment is also dependent on the GTP state of tubulin.
One possible explanation for the observed effects is that the centrosomes in Centrobin-depleted cells behave more like G2 centrosomes than G1/S centrosomes. However, this is unlikely, because EB1/3 asters originating from the centrosome of G2 cells were much more clearly defined than the Cyclin B1-negative asters in the Centrobin-depleted cells. Furthermore, Centrobin-depleted G2 cells were clearly distinguishable from their G1/S counterparts based on the rate of microtubule nucleation and the velocity of the EB3 comets using live cell imaging ( and unpublished observations). An alternative explanation for these effects is that the presence of Centrobin at the daughter centriole is necessary to make it functionally different from the mother centriole. In the absence of Centrobin the daughter centriole may become more like the mother centriole, resulting in increased PCM recruitment and microtubule nucleation. Further studies are required to test this hypothesis.
Centrobin depletion has previously been reported to cause strong disorganization of the microtubule network.Citation6 We also observed a change in microtubule organization concomitant with a decrease in microtubule stability. These changes are most likely due to increased microtubule nucleation, which is the likely consequence of increased PCM recruitment. The increased number of microtubules results in a microtubule network characterized by a large number of short microtubules clustered around the centrosome and a decrease in cortical microtubules. This alteration might also contribute to changes in microtubule stability, because in HeLa cells microtubules are strongly stabilized by interacting with the cortex.Citation18,Citation19
The relationship between the PCM and microtubule nucleation capacity is well established and is observed during the cell cycle, whereby the centrosome recruits large amounts of γ-tubulin during mitosis,Citation20 concomitant with an increase in microtubule nucleation.Citation21 An increase in microtubule nucleation can also be induced by depletion of SZY-20 in C. elegans concomitant with an increase in PCM recruitment and mitotic defects.Citation3 In this regard, Centrobin may be considered analogous to SZY-20, except that SZY-20 inhibits centriole duplication rather than promoting it as Centrobin does.
In summary, our data reveal a novel role for Centrobin in limiting PCM recruitment. Furthermore, it underline the importance of regulating PCM recruitment, as evidenced by the changes observed in multiple centrosome-associated processes including microtubule nucleation and organization. Further elucidation of these mechanisms will provide valuable insights into how a single cellular structure, the centrosome, can control different aspects of cell structure and function.
Materials and Methods
Antibodies and reagents
Centrobin antibody and shRNAs have previously been described.Citation7 The PCM-1 antibody was from Dr A. Merdes,Citation22 AKAP450 and Kendrin antibodies from Dr M. TakahashiCitation10 and Centrin antibody from Dr J. Salisbury.Citation23 Acetylated tubulin antibodies were from Sigma, EB1/3 antibody (clone 15H11) from Absea and Alexa488-labeled TfR1 (T13342) from Life Technologies.
Cell culture and transfections
HeLa cells were grown in RPMI media containing 10% FBS. mCherry-α-tubulin plasmidCitation24 was a gift from Dr R. Tsien. EB3-GFP has been described previously.Citation25 For transfections, 107 cells were mixed with 15 μg DNA and electroporated with 240 V for 10 min.
GFP-Centrobin bacterial artificial chromosome
The BAC RP23-334K19, harboring mouse Centrobin, was obtained from the BACPAC Resources Center (http://bacpac.chori.org). The N-terminal BAC tagging cassette (NLAP: GFP-PreScission-S-peptide-TEV) was PCR amplified using primers that carry 50 nucleotides of homology to the N terminus of Centrobin. Recombineering and stable transfection of the modified BAC was performed as described.Citation26 Briefly, a plasmid carrying two recombinases and the purified tagging cassette were sequentially introduced into the E. coli strain containing the BAC vector using electroporation. Precise incorporation of the tagging cassette was confirmed by PCR and sequencing. Next, the GFP-tagged BAC was isolated from bacteria using the Nucleobond PC100 kit (Macherey-Nagel). Subsequently, HeLa Kyoto cells were transfected using Effectene (Qiagen) and cultivated in selection media containing 400 µg/ml geneticin (G418, Invitrogen). Finally, the pool of HeLa cells stably expressing GFP-Centrobin was analyzed by western blot and immunofluorescence using an anti-GFP antibody (Roche) to verify correct protein size and localization of the tagged transgene.
Western blotting
Cells were resuspended in universal immunoprecipitation buffer (50 mM TRIS-HCl pH 7.4, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 25 mM β-glycerophosphate, 0.2% Triton X-100 and 0.3% NP40, 25 mM NaF, 25 mM Na3VO4, protease inhibitor cocktail) and sonicated for 10 sec, then centrifugated at 15,700 g at 4°C for 30 min, and the supernatant was collected. Western blotting was performed as described previously.Citation7 Membranes were developed using Western Blot Chemiluminescence Reagent Plus in a FujiFilm LAS-3000 Imaging System (Fuji Photo Film).
Immunofluorescence and microscopy
Cells were fixed and permeabilized in ice-cold methanol for 20 min or paraformaldehyde followed by 0.2% TritonX-100 for 10 min each. Cells were blocked with 3% bovine serum albumin in PBS for 30 min, incubated with primary antibody for 50 min, washed three times in PBS, incubated with secondary antibody for 50 min, washed three times in PBS and mounted. Cells were visualized using a DeltaVision personal DV deconvolution microscope (API) and analyzed using softWoRx version 3.6.1 (API).
Live cell imaging
Live cell imaging of cells stably-expressing GFP-Centrobin was performed on an inverted research microscope Nikon Eclipse Ti-E microscope (Nikon) with perfect focus system (PFS) (Nikon), equipped with a Nikon CFI Apo TIRF 100× 1.49 N.A. oil objective (Nikon), a QuantEM 512SC EMCCD camera (Roper Scientific) and controlled with MetaMorph 7.5 software (Molecular Devices). The 16-bit images were projected onto the CCD chip with intermediate lens 2.5× (Nikon C mount adaptor 2.5×) at a magnification of 0.065 μm/pixel. To keep cells at 37°C a stage top incubator (model INUG2E-ZILCS, Tokai Hit) was used.
The microscope was equipped with TIRF-E motorized TIRF illuminator, modified by Roper Scientific France/PICT-IBiSA, Institut Curie for the attachment of the FRAP system (see below). For regular imaging we used a 491 nm 50 mW Calypso (Cobolt) laser for excitation. We used the ET-mCherry filter set (49008, Chroma) for imaging of proteins tagged with mCherry.
FRAP assays were performed using FRAP scanning system I-Las/I-Launch (Roper Scientific France/PICT-IBiSA, Institut Curie) installed on the same microscope and utilizing the lasers mentioned above. For FRAP, we used 100% of the laser power.
For imaging of the EB3-GFP, we used a CSU-X1-A1 Spinning Disc (Yokogawa), equipped with 405-491-561 triple band mirror and GFP emission filters (Chroma). The spinning disc was installed on an inverted Nikon Eclipse Ti-E microscope (Nikon), almost identical to the one described above (the same objective, camera, software, filters and lasers and heating device). The only difference was that this microscope was equipped with custom-ordered illuminator (Nikon, MEY10021) for the attachment of the FRAP scanning system I-Las/I-Launch (Roper Scientific France/PICT-IBiSA, Institut Curie). The 16-bit images were projected onto the CCD chip with intermediate lens 2.0× (Edmund Optics) at a magnification of 0.068 μm/pixel.
Microtubule assays
To assay microtubule regrowth cells were placed in cold medium and on ice for 30 min to denature microtubules, then returned to warm (37°C) media for the appropriate time (0–10 min). Cells were fixed with ice-cold methanol, stained with α-tubulin antibody and imaged as described above.
To assay microtubule stability cells were treated with 10 μM nocodazole for 30 min to depolymerize the microtubule network. Cells were fixed with ice-cold methanol, stained with α-tubulin antibody and imaged as described above.
Statistical analysis
Statistical analysis was performed using the Mann-Whitney U-test for the microtubule dynamics data in Table S1 or a two-tailed t-test for all other experiments.
Additional material
Download Zip (8.3 MB)Acknowledgments
We wish to thank Dr J. Salisbury for the gift of the Centrin antibody, Dr A. Merdes for the gift of the PCM-1 antibody, Dr M. Takahashi for the gift of the AKAP450 and Kendrin antibodies, Dr R. Tsien for the gift of mCherry-α-tubulin plasmid and Dr C. Moores for critical reading of the manuscript. K.K.K. is a NHMRC Senior Principal Research Fellow and this work is supported in part by a Program grant from the National Health and Medical Research Council of Australia to K.K.K. A.A. and I.G. were supported by the Netherlands Organization for Scientific Research ALW-VICI and ZonMw-TOP grants. Work of I.P. at the lab of Tony Hyman is supported by the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreement 241548 (MitoSys Project).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof Y-D, Nigg EA. Plk4-induced centriole biogenesis in human cells. Dev Cell 2007; 13:190 - 202; http://dx.doi.org/10.1016/j.devcel.2007.07.002; PMID: 17681131
- Carvalho-Santos Z, Machado P, Branco P, Tavares-Cadete F, Rodrigues-Martins A, Pereira-Leal JB, et al. Stepwise evolution of the centriole-assembly pathway. J Cell Sci 2010; 123:1414 - 26; http://dx.doi.org/10.1242/jcs.064931; PMID: 20392737
- Song MH, Aravind L, Müller-Reichert T, O’Connell KF. The conserved protein SZY-20 opposes the Plk4-related kinase ZYG-1 to limit centrosome size. Dev Cell 2008; 15:901 - 12; http://dx.doi.org/10.1016/j.devcel.2008.09.018; PMID: 19081077
- Bobinnec Y, Khodjakov A, Mir LM, Rieder CL, Eddé B, Bornens M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J Cell Biol 1998; 143:1575 - 89; http://dx.doi.org/10.1083/jcb.143.6.1575; PMID: 9852152
- Zou CZ, Li J, Bai YJ, Gunning WT, Wazer DE, Band V, et al. Centrobin: a novel daughter centriole-associated protein that is required for centriole duplication. J Cell Biol 2005; 171:437 - 45; http://dx.doi.org/10.1083/jcb.200506185; PMID: 16275750
- Jeong Y, Lee J, Kim K, Yoo JC, Rhee K. Characterization of NIP2/centrobin, a novel substrate of Nek2, and its potential role in microtubule stabilization. J Cell Sci 2007; 120:2106 - 16; http://dx.doi.org/10.1242/jcs.03458; PMID: 17535851
- Jeffery JM, Urquhart AJ, Subramaniam VN, Parton RG, Khanna KK. Centrobin regulates the assembly of functional mitotic spindles. Oncogene 2010; 29:2649 - 58; http://dx.doi.org/10.1038/onc.2010.37; PMID: 20190801
- Webster DR, Borisy GG. Microtubules are acetylated in domains that turn over slowly. J Cell Sci 1989; 92:57 - 65; PMID: 2674164
- Ou Y, Rattner JB. The centrosome in higher organisms: structure, composition, and duplication. Int Rev Cytol 2004; 238:119 - 82; http://dx.doi.org/10.1016/S0074-7696(04)38003-4; PMID: 15364198
- Takahashi M, Yamagiwa A, Nishimura T, Mukai H, Ono Y. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol Biol Cell 2002; 13:3235 - 45; http://dx.doi.org/10.1091/mbc.E02-02-0112; PMID: 12221128
- Wiese C, Zheng YX. Microtubule nucleation: gamma-tubulin and beyond. J Cell Sci 2006; 119:4143 - 53; http://dx.doi.org/10.1242/jcs.03226; PMID: 17038541
- Gregoretti IV, Margolin G, Alber MS, Goodson HV. Insights into cytoskeletal behavior from computational modeling of dynamic microtubules in a cell-like environment. J Cell Sci 2006; 119:4781 - 8; http://dx.doi.org/10.1242/jcs.03240; PMID: 17093268
- Gliksman NR, Skibbens RV, Salmon ED. How the transition frequencies of microtubule dynamic instability (nucleation, catastrophe, and rescue) regulate microtubule dynamics in interphase and mitosis: analysis using a Monte Carlo computer simulation. Mol Biol Cell 1993; 4:1035 - 50; PMID: 8298190
- Vorobjev I, Maly I. Microtubule length and dynamics: Boundary effect and properties of extended radial array. Cell Tissue Biol 2008; 2:272 - 81; http://dx.doi.org/10.1134/S1990519X08030085
- Mitchison TJ, Kirschner MW. Some thoughts on the partitioning of tubulin between monomer and polymer under conditions of dynamic instability. Cell Biophys 1987; 11:35 - 55; PMID: 2450668
- Gudi R, Zou C, Li J, Gao Q. Centrobin-tubulin interaction is required for centriole elongation and stability. J Cell Biol 2011; 193:711 - 25; http://dx.doi.org/10.1083/jcb.201006135; PMID: 21576394
- Gopalakrishnan J, Chim YC, Ha A, Basiri ML, Lerit DA, Rusan NM, et al. Tubulin nucleotide status controls Sas-4-dependent pericentriolar material recruitment. Nat Cell Biol 2012; 14:865 - 73; http://dx.doi.org/10.1038/ncb2527; PMID: 22729084
- Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, et al. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol 2005; 168:141 - 53; http://dx.doi.org/10.1083/jcb.200405094; PMID: 15631994
- Lansbergen G, Akhmanova A. Microtubule plus end: a hub of cellular activities. Traffic 2006; 7:499 - 507; http://dx.doi.org/10.1111/j.1600-0854.2006.00400.x; PMID: 16643273
- Khodjakov A, Rieder CL. The sudden recruitment of gamma-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J Cell Biol 1999; 146:585 - 96; http://dx.doi.org/10.1083/jcb.146.3.585; PMID: 10444067
- Piehl M, Tulu US, Wadsworth P, Cassimeris L. Centrosome maturation: measurement of microtubule nucleation throughout the cell cycle by using GFP-tagged EB1. Proc Natl Acad Sci USA 2004; 101:1584 - 8; http://dx.doi.org/10.1073/pnas.0308205100; PMID: 14747658
- Dammermann A, Merdes A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J Cell Biol 2002; 159:255 - 66; http://dx.doi.org/10.1083/jcb.200204023; PMID: 12403812
- Salisbury JL, Suino KM, Busby R, Springett M. Centrin-2 is required for centriole duplication in mammalian cells. Curr Biol 2002; 12:1287 - 92; http://dx.doi.org/10.1016/S0960-9822(02)01019-9; PMID: 12176356
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 2004; 22:1567 - 72; http://dx.doi.org/10.1038/nbt1037; PMID: 15558047
- Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, et al. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J Neurosci 2003; 23:2655 - 64; PMID: 12684451
- Poser I, Sarov M, Hutchins JRA, Hériché J-K, Toyoda Y, Pozniakovsky A, et al. BAC TransgeneOmics: a high-throughput method for exploration of protein function in mammals. Nat Methods 2008; 5:409 - 15; http://dx.doi.org/10.1038/nmeth.1199; PMID: 18391959