The differentiation of human embryonic stem cells (hESCs) into hematopoietic stem cells (HSCs) that match the repopulation potential of bone marrow-derived correlates has tremendous clinical promise, but this goal has eluded researchers despite 15 y of dogged effort. In vivo, HSCs have been shown to arise from specialized endothelial cells (ECs) with hemogenic potential, and endothelial to hematopoietic transition (EHT) has also been demonstrated in vitro using a mouse ESC platform (reviewed in ref. Citation1). Recently, we generated transgenic hESCs in which endothelial and early hematopoietic identity are demarcated by expression of distinct fluorescent reporter genes, and used this tool to observe the phenotypic progression of hESC-derived hemogenic ECs during and after EHT.Citation2 Here, we discuss some of the insights gained from our dual reporting transgenic cell line and address the remaining obstacles facing generation of definitive HSCs from hESCs.
While EHT has been noted at multiple developmental time-points and within distinct embryonic tissue primordiae, hemogenic ECs that generate definitive HSCs have only been clearly demonstrated in the aorta-gonad-mesonephros (AGM) regionCitation1 and, recently, in the e10.5–11.5 mouse head.Citation3 Considering the scarcity of these specialized vascular beds within the embryo, the inability to generate definitive HSCs from hESCs could arise from at least two failings: either the specific AGM-type (or Head-type) hemogenic ECs that account for HSCs with definitive potential do not arise for lack of important inductive factors that are absent during differentiation; and/or there is an imbalance in the niche derived factors that support or instruct self-renewal of newly emerging HSCs. Using a hESC-based system, we showed that both intrinsic and niche derived factors govern lineage potential of hemogenic ECs and early hematopoietic progenitors.Citation2 We demonstrated expansion of lineage potential with advancing stages of hESC differentiation, from progenitors that are biased toward megakaryocytic/erythropoietic lineages, to later stage progenitors with myeloid potential; and we identified a mechanism by which Notch ligands derived from the microenvironment direct multipotent hematopoietic progenitors toward myeloid fate.
Our demonstration of discreet hemogenic EC sub-populations mirrors in vivo hemogenesis, in which a sequence of hematopoietic programs is initiated at different temporal and anatomical coordinates. Yet while the more advanced population of hemogenic ECs obtained in our study shared qualities of intra-embryonic erythroid/myeloid progenitors, we did not generate cells that exhibited properties of definitive HSCs; attempts to engraft hESC-derived hematopoietic cells into immune-compromised mice failed, and efforts to generate lymphoid cells in vitro were unsuccessful.Citation2 Recent studies have detected T cell potential from hESC-derived hematopoietic progenitors,Citation4 suggesting their ability to establish a definitive HSC program, however, functional equivalency of hESC-derived T cells and their bone marrow-derived correlates has not been demonstrated. Moreover, in vivo engraftment and serial repopulation of mouse or human ESC derived hematopoietic cells remains elusive. Given the scarcity of hemogenic ECs that transition to definitive HSCs in vivo, the spontaneous induction of an equipotent hemogenic EC population from hESCs is likely to be an extremely rare event, so imaging this transition may facilitate isolation and study of hematopoietic progenitors at a clonal level. In mice, a transgenic reporter based approach has resolved two distinct phenotypes of hemogenic EC: Tie2+ cells that generate myeloid restricted hematopoietic progenitors, and Sca1+ cells that give rise to definitive HSCs.Citation5 Segregation of hESC-derived hemogenic EC populations based on similar phenotypes may enable identification of cells with definitive HSC potential. However, a human homolog of Sca1+ does not exist, so surrogate markers that distinguish AGM-type hemogenic ECs must first be determined.
Even if hemogenic ECs with definitive potential are specified from hESCs in vitro, albeit at a exceedingly low frequency, the influence of niche-derived signals must be taken into account. Vascular ECs, both in vitro and in vivo,Citation6 are a source of paracrine factors that affect homeostasis of hematopoietic progenitors. We showed that Dll4 is strongly expressed in primary and hESC-derived ECs, and that Notch receptor activation by Dll4 directs hESC-derived hematopoietic progenitors toward myeloid fate.Citation2 This contrasts with adult hematopoiesis, where Notch activation has been shown to inhibit myelopoiesis and promote lymphoid differentiation, and previous studies have employed stroma that constitutively express Dll1 to generate T-cells from hESC-derived hematopoietic progenitors.Citation7 Additionally, Notch has been shown to be critical for the definitive phase of hemogenesis; mice lacking the Notch1 receptor are incapable of generating arterial ECs and definitive HSCs, and loss of Jagged1 results in loss of definitive HSCs without affecting induction of arterial ECs (reviewed in ref. Citation8). Thus, activation of Notch may serve diverse functions depending of developmental context and the ability of Dll4 to promote myeloid fate in our system may be unique to intermediate stage erythroid/myeloid-restricted progenitors. Parsing the specific function of Notch ligands and receptors at sequential phases of developmental hemogenesis will be essential to establishing growth conditions that support rare HSCs that emerge during hESC differentiation. Hence, the use of transgenic cell lines that demarcate distinct hemogenic EC sub-populations during hESC differentiation could play a critical role in identification and maintenance of therapeutically useful hematopoietic progenitors. ()
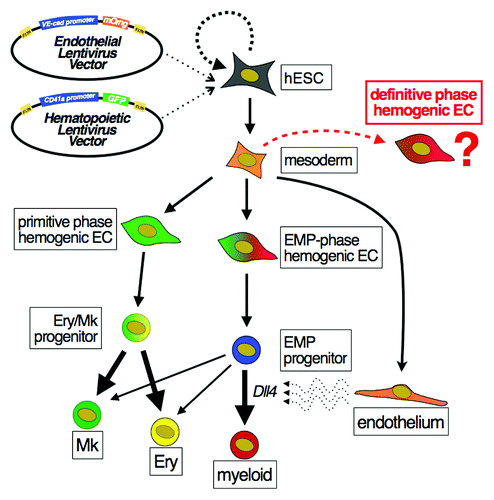
Figure 1. Human embryonic stem cells recapitulate distinct waves of developmental hemogenesis. A dual reporting transgenic hESC line was generated using promoter fragments that are specifically activated in endothelial (VE-cadherin) or early hematopoietic (CD41a) cells. Monitoring of reporter activity and single cell lineage tracking identified distinct waves of primitive hemogenic ECs that were biased to erythroid and megakaryocytic fate, and later stage erythroid/myeloid progenitors (EMPs). Expression of Dll4 on vascular feeder cells specifically promoted myelopoiesis from multipotent EMPs. The capacity for hESCs to generate hemogenic ECs with definitive potential remains to be determined.
References
- Hirschi KK. Hemogenic endothelium during development and beyond. Blood 2012; 119:4823 - 7; http://dx.doi.org/10.1182/blood-2011-12-353466; PMID: 22415753
- Rafii S, Kloss CC, Butler JM, Ginsberg M, Gars E, Lis R, et al. Human ESC-derived hemogenic endothelial cells undergo distinct waves of endothelial to hematopoietic transition. Blood 2013; 121:770 - 80; http://dx.doi.org/10.1182/blood-2012-07-444208; PMID: 23169780
- Li Z, Lan Y, He W, Chen D, Wang J, Zhou F, et al. Mouse embryonic head as a site for hematopoietic stem cell development. Cell Stem Cell 2012; 11:663 - 75; http://dx.doi.org/10.1016/j.stem.2012.07.004; PMID: 23122290
- Kennedy M, Awong G, Sturgeon CM, Ditadi A, LaMotte-Mohs R, Zúñiga-Pflücker JC, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep 2012; 2:1722 - 35; http://dx.doi.org/10.1016/j.celrep.2012.11.003; PMID: 23219550
- Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Inagawa T, et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 2011; 9:541 - 52; http://dx.doi.org/10.1016/j.stem.2011.10.003; PMID: 22136929
- Butler JM, Rafii S. Generation of a vascular niche for studying stem cell homeostasis. Methods Mol Biol 2012; 904:221 - 33; PMID: 22890935
- Timmermans F, Velghe I, Vanwalleghem L, De Smedt M, Van Coppernolle S, Taghon T, et al. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J Immunol 2009; 182:6879 - 88; http://dx.doi.org/10.4049/jimmunol.0803670; PMID: 19454684
- Bigas A, Espinosa L. Hematopoietic stem cells: to be or Notch to be. Blood 2012; 119:3226 - 35; http://dx.doi.org/10.1182/blood-2011-10-355826; PMID: 22308291