Cancer recurrence is associated with treatment failure and is the main cause of death,Citation1 and thus remains to be a major clinical challenge. Elevated level of the cyclin-dependent kinase 1 (CDK1) correlates with cancer recurrence and treatment resistance in patients with breast cancer or colorectal cancer.Citation2,Citation3 It is proposed that defects in CDKs may lead to the accumulated genetic defects, which render cells less sensitive to drug-induced growth inhibition and apoptosis.Citation4 CDK1 is the most essential CDK, as it alone is sufficient to drive cell division cycle in mammalian cells.Citation4 CDK1 in complex with B-type cyclins regulates the mitotic entry of the cell cycle. Once DNA damages occur, CDK1/cyclin B1 becomes inactivated, and cells are prevented from entering mitosis until the damages are repaired. CDK1 is inactivated by the nuclear vs. cytoplasmic kinases Wee1 and Myt1, which phosphorylate CDK1 on its tyrosin-14 and -15 sites.Citation5 While it is activated by the antagonist of Wee1 and Myt1, a family of phosphatases of Cdc25A, B and C dephosphorylate CDK1 on the same tyrosin-sites served for Wee1 and Myt1.Citation6 The proper level and activity of CDK1 is thus kept in balance by these kinases and phosphatases that are involved in the cell cycle progression (). However, the role of CDK1 and the regulation of its phosphorylation and subcellular localization upon cellular response to chemotherapeutic drugs are poorly understood.
Figure 1. To regulate the proper entry of mitosis, CDK1 is inhibited by Wee1 which phosphorylate CDK1, while it is activated Cdc25A and C, which dephosphorylate CDK1. Hedblom et al. for the first time showed that RARγ and CDK1 form a reciprocal regulatory circuit and influence the function and level of P27kip protein, and control the G0/G1 phases of cell cycle.
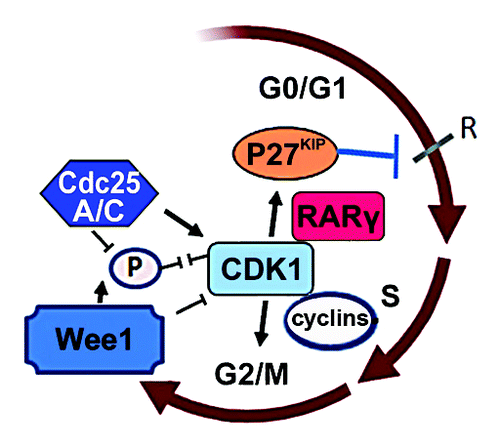
In a recent issue of Cell Cycle, Hedblom et al.Citation7 showed that altered level of CDK1 is associated with disease recurrence and poor overall survival of patients with acute myeloid leukemia. These novel findings indicate that CDK1 is a critical factor mediating cellular response to ATRA treatment. Only elimination of CDK1, not CDK2, causes a decreased proportion of G0/G1 cells and a concomitant increase in mitotic cells, suggesting that cells without proper level of CDK1 had accelerated mitosis. Defects in CDK1 expression level thus confer U-937 leukemic cells to be less sensitive to all trans retinoic acid (ATRA)-induced G0/G1 cell cycle arrest and differentiation. Hedblom et al.Citation7 demonstrate that defects in CDK1 expression cause alterations in the expression levels and activities of several proteins, which are the key regulators for cell growth and survival. These include: (1) elimination of CDK1 in U-937 cells leads to a significant reduction in the level of P27kip, a key regulator for G0/G1 checkpoint; (2) elimination of CDK1 in U-937 cells also results in an increased level of phosphorylated Akt, a key survival factor. These alterations are likely linked to the CDK1-mediated resistance to ATRA treatment.
Hedblom et al. have unravelled several novel mechanisms on how the phosphorylation and subcellular localization of CDK1 is regulated upon cellular response to ATRA. Thus, ATRA receptor RARγ, (not RARα) is required for the regulation of CDK1 expression, phosphorylation and protein stability upon induction of ATRA. RARγ regulates CDK1 level and activity through a direct formation of protein-protein complex in the nucleus of U-937 cells and F9 cells. In the absence of RARγ, ATRA is unable to downregulate CDK1 expression and phosphorylation. This suggests that RARγ is a critical factor to balance the CDK1 level and activity to ensure ATRA to achieve optimal effects on cancer cells. Similar to what is observed in U-937 cells with defects in CDK1 expression; tumor cells with defects in RARγ also display a reduced level of P27kip. Thus, RARγ and CDK1 may form a reciprocal regulatory circuit and influence the function and level of P27kip protein ().
Hedblom et al.Citation7 show that ATRA treatment results in a reduced the level of Wee1 kinase and Cdc25A phosphatase in the nucleus, which coincides with the decreased level of nuclear CDK1. This suggests that ATRA inhibits CDK1 activity in the nucleus by reducing Cdc25A phosphatase, the activator that is responsible for CDK1 activity. It is interesting to note that ATRA treatment also reduces the level of Wee1 kinase, which acts as the inhibitor for CDK1. This suggests that despite that the downregulation of CDK1 is required for ATRA to achieve the optimal effect, a proper level and activity of CDK1 need to be maintained and kept in balance by Wee1 kinase and CDC25A. The novel findings suggest that the regulation of CDK1 is cooperatively mediated by the cell cycle regulators Wee1 kinase and CDC25A and the hormone receptor RARγ in response to ATRA treatment.
Retinoid-based therapies are increasingly being utilized to treat various types of cancers. ATRA represents a class of anticancer drug that can induce tumor cells to differentiate and restore their normal function. Because CDK1 is a downstream effect protein of multiple pathways, including Wee1, and Cdc25A and Cdc25C and RARγ, the proper regulation of its expression and activity is essential for sensitizing the cells to respond to treatment. The study by Hedblom et al.Citation7 suggests that CDK1 and its associated regulators are the ideal targets for cancer therapy, and their novel findings highlight the therapeutic potential using ATRA for targeting CDK1 and its associated proteins in cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kröger N. Approaches to relapse after allogeneic stem cell transplantation. Curr Opin Oncol 2011; 23:203 - 8; http://dx.doi.org/10.1097/CCO.0b013e328342c6c8; PMID: 21157340
- Kim SJ, Nakayama S, Shimazu K, Tamaki Y, Akazawa K, Tsukamoto F, et al. Recurrence risk score based on the specific activity of CDK1 and CDK2 predicts response to neoadjuvant paclitaxel followed by 5-fluorouracil, epirubicin and cyclophosphamide in breast cancers. Ann Oncol 2012; 23:891 - 7; http://dx.doi.org/10.1093/annonc/mdr340; PMID: 21821547
- Zeestraten EC, Maak M, Shibayama M, Schuster T, Nitsche U, Matsushima T, et al. Specific activity of cyclin-dependent kinase I is a new potential predictor of tumour recurrence in stage II colon cancer. Br J Cancer 2012; 106:133 - 40; http://dx.doi.org/10.1038/bjc.2011.504; PMID: 22108518
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 2009; 9:153 - 66; http://dx.doi.org/10.1038/nrc2602; PMID: 19238148
- McGowan CH, Russell P. Cell cycle regulation of human WEE1. EMBO J 1995; 14:2166 - 75; PMID: 7774574
- Boutros R, Lobjois V, Ducommun B. CDC25 phosphatases in cancer cells: key players? Good targets?. Nat Rev Cancer 2007; 7:495 - 507; http://dx.doi.org/10.1038/nrc2169; PMID: 17568790
- Hedblom A, Laursen KB, Miftakhova R, Sarwar M, Anagnostaki L, Bredberg A, et al. CDK1 interacts with RARγ and plays an important role in treatment response of acute myeloid leukemia. Cell Cycle 2013; 12:1251 - 66; http://dx.doi.org/10.4161/cc.24313; PMID: 23518499