Keywords: :
Epidermal growth factor receptor (EGFR) is highly expressed or overly active in many types of cancer, including head and neck, breast, ovarian, esophageal and non-small cell lung cancers.Citation1 Increased expression of EGFR has been associated with resistance to standard therapies and poor patient prognosis. EGFR signaling promotes cancer progression by stimulating angiogenesis, cancer cell proliferation, migration, invasion and metastasis. To design more efficient anti-EGFR therapies for cancers, the mechanisms by which EGFR expression and signaling are modulated must be well defined.
Phosphoinositides control EGFR signaling during the endocytosis, endosomal sorting and lysosomal degradation of EGFR.Citation2,Citation3 It is well established that PI4,5P2 is abundant at the plasma membrane and is required for clathrin-dependent endocytosis of membrane receptors,Citation4 while PI3P is abundant at endosomal membranes, where it recruits PI3P binding proteins that are essential in endosomal sorting of receptors.Citation4 Though PI4,5P2 is present at endosomes, its role in endosomal sorting is unexpected and was largely ignored in previous work. Recently, we have demonstrated that type Igamma phosphatidylinositol phosphate kinase i5 (PIPKIγi5), an enzyme that generates PI4,5P2, is targeted to endosomes and is required for the endosomal sorting and lysosomal degradation of EGFR.Citation5 These findings challenge the dogma that PI4,5P2 primarily functions in endocytosis, while PI3P controls endosomal sorting.
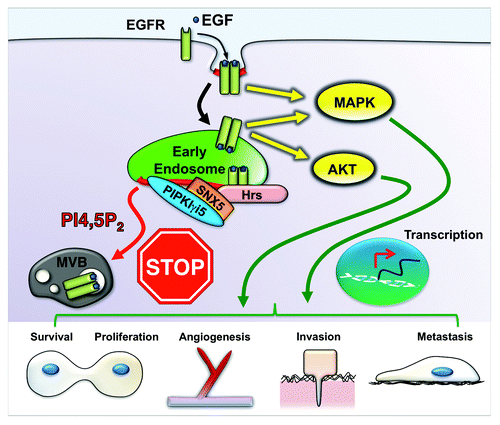
PIPKIγ is a major enzyme that synthesizes PI4,5P2 in the cell.Citation6 Six PIPKIγ variants, known as PIPKIγi1–i6, have been identified in humans.Citation7 They are sequence identical in their N terminus and kinase domain, but each isoform has a unique extension at the C terminus, which mediates their targeting and interaction with distinct effector proteins. For example, talin and the exocyst complex form a unique interaction with PIPKIγi2, which modulates adhesion turnover and cell polarization and is required for EGF-induced directional migration of cancer cells and could modulate cancer metastasis.Citation7 Alternatively, PIPKIγi5 controls EGFR endosomal sorting and degradation, as shown in . PIPKIγi5 specifically interacts with Sorting Nexin 5 (SNX5), an endosomal PI4,5P2 effector. At endosomes, production of PI4,5P2 by PIPKIγi5 is required for the interaction of SNX5 with Hrs, a key subunit of the endosomal sorting complex required for transport -0 (ESCRT-0) that binds and mediates the sorting of EGFR from the limiting membrane to intraluminal vesicles (ILVs) of the multivesicular body (MVB). The SNX5-Hrs interaction protects Hrs from ubiquitination, a modification that inhibits Hrs function. Thus, PIPKIγi5 and SNX5 are required for a strong interaction of Hrs with ubiquitinated EGFR and facilitate Hrs-mediated EGFR intraluminal sorting. This process is critical for the termination of EGFR signaling and degradation of EGFR at lysosome. Loss of either PIPKIγi5 or SNX5 leads to the accumulation of activated EGFR at the limiting membrane of endosomes, where EGFR continues to signal and cannot be degraded. This results in highly enhanced and prolonged EGFR signaling, including ERK and AKT activation, which also correlates with cancer progression. The functions of PIPKIγi2 and PIPKIγi5 in EGFR-mediated cell migration and EGFR signaling suggest potential roles of PIPKIγ in cancer progression. Changes in alternative splicing for PIPKIγ in cancer may affect cancer progression. For instance, an increase of PIPKIγi2 expression, which enhances migration, and decrease of PIPKIγi5 expression, which enhances EGFR signaling, could correlate with cancer progression.
Figure 1. PIPKIγi5 controls EGFR signaling. To control EGFR signaling, the activated receptor is rapidly ubiquitinated and endocytosed to endosomes. The receptor continues to signal at the limiting membrane of endosomes until it is sorted into ILVs of MVB. This process requires PIPKIγi5 and SNX5 to coordinate with Hrs in the ESCRT complex to mediate intraluminal sorting of the receptor. Loss of PIPKIγi5 or SNX5 results in enhanced and prolonged EGFR signaling. This increased signaling of EGFR often occurs in cancers and leads to enhanced angiogenesis, cancer cell survival, proliferation, invasion and metastasis.
Therapeutic anti-EGFR monoclonal antibodies, such as cetuximab and panitumumab, have been used as a therapy to treat cancers. The interaction of the monoclonal antibodies with membrane EGFR stimulates the endocytosis and lysosomal degradation of EGFR.Citation8 But many cancers are resistant to this therapy. Loss of the PIPKIγi5 signaling nexus could block the lysosomal trafficking and degradation of EGFR. It provides a possible mechanism of resistance to anti-EGFR monoclonal antibody therapy. The PIPKIγi5 signaling nexus could potentially be manipulated to promote the degradation of EGFR and terminate its signaling with significant clinical implications.
The function of PIPKIγi5 signaling nexus in endosomal trafficking is receptor-selective. It suggests that there are distinct pathways that control endosomal trafficking and degradation for different subsets of receptors. Further studies can explain how the endosomal sorting of specific receptors is modulated dependently or independently of PIPKIγi5. It will broaden the understanding of PIPKIγi5 in cancer progression by defining the specific receptors, beyond EGFR, regulated by this pathway, and explain how the collective signaling pathways contribute toward pathogenic processes in cancer.
References
- Arteaga CL. Epidermal growth factor receptor dependence in human tumors: more than just expression?. Oncologist 2002; 7:Suppl 4 31 - 9; http://dx.doi.org/10.1634/theoncologist.7-suppl_4-31; PMID: 12202786
- Barbieri MA, Heath CM, Peters EM, Wells A, Davis JN, Stahl PD. Phosphatidylinositol-4-phosphate 5-kinase-1beta is essential for epidermal growth factor receptor-mediated endocytosis. J Biol Chem 2001; 276:47212 - 6; http://dx.doi.org/10.1074/jbc.C100490200; PMID: 11581249
- Petiot A, Faure J, Stenmark H, Gruenberg J. PI3P signaling regulates receptor sorting but not transport in the endosomal pathway. J Cell Biol 2003; 162:971 - 9; http://dx.doi.org/10.1083/jcb.200303018; PMID: 12975344
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006; 443:651 - 7; http://dx.doi.org/10.1038/nature05185; PMID: 17035995
- Sun Y, Hedman AC, Tan X, Schill NJ, Anderson RA. Endosomal Type Iγ PIP 5-Kinase Controls EGF Receptor Lysosomal Sorting. Dev Cell 2013; 25:144 - 55; http://dx.doi.org/10.1016/j.devcel.2013.03.010; PMID: 23602387
- Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol 2003; 194:77 - 89; http://dx.doi.org/10.1007/s00232-003-2027-7; PMID: 14502432
- Sun Y, Thapa N, Hedman AC, Anderson RA. Phosphatidylinositol 4,5-bisphosphate: Targeted production and signaling. Bioessays 2013; 35:513 - 22; http://dx.doi.org/10.1002/bies.201200171; PMID: 23575577
- Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets 2012; 16:15 - 31; http://dx.doi.org/10.1517/14728222.2011.648617; PMID: 22239438