Formation of a functional vascular system is critical for the delivery of nutrients, the removal of waste, and gas exchange. During vasculogenesis, the de novo formation of blood vessels, mesodermal cells differentiate into endothelial cell precursors that proliferate and migrate to specified locations in the embryo before assembling into cord-like structures to form the primary vascular plexus.Citation1 Redistribution of junctional molecules, establishment of apicobasal polarity, and cell morphology changes all facilitate the opening of vessel lumens. These primitive blood vessels are further pruned and remodeled by angiogenesis when new vascular branches form by sprouting from pre-existing vessels. Additionally, endothelial cells become specified to contribute to either the venous or arterial vasculature.Citation1 Understanding the molecular and cellular mechanisms underlying endothelial cell behavior will enable us to develop more efficacious therapies for diseases, such as atherosclerosis, rheumatoid arthritis, and tumorigenesis.
The specification of the cardiovascular lineage and the subsequent morphogenesis of the heart and vessels depend on the combined activities of a number of transcription factors. Work within our lab revealed a novel role for the transcription factor CASTOR (CASZ1) in Xenopus cardiogenesis by regulating cardiomyocyte differentiation.Citation2 In a recent study, we also showed that CASZ1 is required for blood vessel branching and lumen formation in Xenopus embryos, independent of its role in cardiac development.Citation3 At the cellular level, depletion of CASZ1 in primary human endothelial cells results in impaired adhesion to the underlying substrate, aberrant contractility, and G1/S cell cycle arrest, indicating that CASZ1 is necessary for promoting endothelial cell behaviors associated with proper vascular assembly.Citation3 Utilizing a chromatin immunoprecipitation-cloning screen, we found that CASZ1 modulates these endothelial cell behaviors by activating the expression of its direct transcriptional target, epidermal growth factor-like domain 7 (EGFL7). Depletion of EGFL7, an endothelial-secreted extracellular matrix (ECM) protein, resulted in poorly arborized vascular networks that were devoid of vessel lumens, indicating a requirement for EGFL7 in angiogenesis and lumen morphogenesis in accordance with previous reports.Citation4,Citation5 Moreover, the EGFL7-deficient vascular networks were similar to those in CASZ1-depleted embryos. We linked this CASZ1/EGFL7 transcriptional hierarchy to the RhoA GTPase signaling pathway, which directly controls the cellular outputs we observed to be defective under CASZ1 and EGFL7-depleted conditions. RhoA transcript levels and activity were significantly diminished in the absence of either CASZ1 or EGFL7. Consequently, formation of actin-based stress fibers and localization of focal adhesion markers at sites of substrate contact were aberrant in CASZ1-depleted cells, but these defects were rescued by reintroduction of EGFL7.
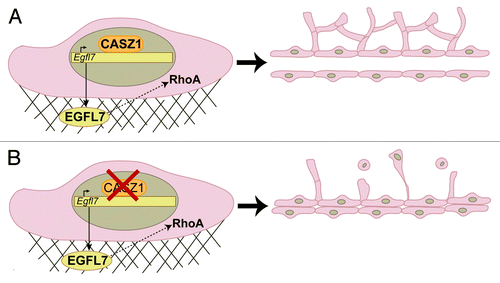
From these results, we propose a model whereby CASZ1 binds to and activates EGFL7 gene expression in endothelial cells in order to release EGFL7 into the ECM. Through yet unknown mechanisms, we hypothesize that EGFL7 binds to cell-surface receptors, such as integrins, to activate a signaling cascade necessary for RhoA transcription and its subsequent activity. RhoA then directly modulates endothelial cell behaviors, such as adhesion and contractility, to promote the proper assembly of vascular networks (). While we have shown that activation of this pathway is necessary for the formation of a functional vascular system from a developmental standpoint, it is not surprising that improper activation of such a pathway could lead to pathological vascular remodeling in adult tissues, such as during tumorigenesis. While highly expressed in developing vessels, EGFL7 is downregulated in the quiescent vasculature of the adult, but is upregulated again in response to injury or cellular stress.Citation4 Indeed, high EGFL7 levels are correlated with several tumors and cancer cell lines, and EGFL7 monoclonal antibodies are currently being tested in clinical trials for use in vascular tumor therapies (http://www.gene.com/medical-professionals/pipeline).Citation4 RhoA has been shown to be required for lumen formation, but increased RhoA activity also induces vascular permeability, which potentially associates RhoA with the unstable, leaky vasculature characteristic of tumors.Citation6 Therefore, uncovering the molecular networks underlying embryonic development may provide novel targets for the design of therapeutics to treat patients with cancer.
Figure 1. (A) Proper expression and activity of CASZ1 in endothelial cells results in transcriptional activation of Egfl7 and subsequent RhoA activity, thereby promoting the assembly of a well-branched, lumenized vascular system. (B) Disruption of CASZ1 function results in cords of endothelial cells lacking a central lumen and angiogenic sprouts. Branches that are apparent consist of thin, elongated cells that are unable to maintain adhesion to the underlying matrix or existing vasculature.
There have been limited studies on mammalian CASZ1 in both development and disease. Recently, the human ortholog of Casz1 was identified and shown to be highly expressed in adult heart tissue.Citation7 The evolutionary role of CASZ1 in cardiovascular development is further emphasized by a recent genome-wide association study, demonstrating a genetic association between the Casz1 locus and both blood pressure and hypertension.Citation8 However, despite the essential role of CASZ1 in cardiovascular development and human disease, the cellular requirements and molecular mechanisms by which CASZ1 regulates cardiac development remain unknown. To address these issues, we generated a Casz1-knockout mouse that will provide a means to understand mechanistically how this transcription factor functions in cardiovascular disease. Future studies include identifying additional transcriptional targets of CASZ1 in the heart and vasculature and investigating how CASZ1 regulates transcription. To that end, we are undertaking a number of proteomics-based approaches to determine how CASZ1 itself is regulated, to identify cardiac and/or vascular-specific co-factors with which CASZ1 interacts to regulate transcription, and to uncover novel downstream pathways dependent on CASZ1 function.
References
- Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 2011; 12:551 - 64; http://dx.doi.org/10.1038/nrm3176; PMID: 21860391
- Christine KS, Conlon FL. Vertebrate CASTOR is required for differentiation of cardiac precursor cells at the ventral midline. Dev Cell 2008; 14:616 - 23; http://dx.doi.org/10.1016/j.devcel.2008.01.009; PMID: 18410736
- Charpentier MS, Christine KS, Amin NM, Dorr KM, Kushner EJ, Bautch VL, et al. CASZ1 promotes vascular assembly and morphogenesis through the direct regulation of an EGFL7/RhoA-mediated pathway. Dev Cell 2013; 25:132 - 43; http://dx.doi.org/10.1016/j.devcel.2013.03.003; PMID: 23639441
- Nichol D, Stuhlmann H. EGFL7: a unique angiogenic signaling factor in vascular development and disease. Blood 2012; 119:1345 - 52; http://dx.doi.org/10.1182/blood-2011-10-322446; PMID: 22160377
- Nikolic I, Stankovic ND, Bicker F, Meister J, Braun H, Awwad K, et al. EGFL7 ligates αvβ3 integrin to enhance vessel formation. Blood 2013; 121:3041 - 50; http://dx.doi.org/10.1182/blood-2011-11-394882; PMID: 23386126
- Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta 2009; 1796:91 - 8; PMID: 19327386
- Liu Z, Yang X, Tan F, Cullion K, Thiele CJ. Molecular cloning and characterization of human Castor, a novel human gene upregulated during cell differentiation. Biochem Biophys Res Commun 2006; 344:834 - 44; http://dx.doi.org/10.1016/j.bbrc.2006.03.207; PMID: 16631614
- Takeuchi F, Isono M, Katsuya T, Yamamoto K, Yokota M, Sugiyama T, et al. Blood pressure and hypertension are associated with 7 loci in the Japanese population. Circulation 2010; 121:2302 - 9; http://dx.doi.org/10.1161/CIRCULATIONAHA.109.904664; PMID: 20479155