Histone methyltransferases (HMT) are chromatin modifiers that regulate the transcriptomic landscape in normal development as well as in diseases such as cancer.Citation1 Enhancer of Zeste 2 (EZH2), a component of the polycomb repressive complex 2 (PRC2), trimethylates histone 3 lysine 27 (H3K27me3), resulting in a more compact chromatin structure known to repress gene activation.Citation2 Overexpression of EZH2 has been strongly implicated in oncogenesis of many different cancers, including ovarian cancer (OC),Citation2 and increased EZH2 activity has been linked to OC resistance to platinum-based chemotherapy, presumably by inhibiting crucial tumor suppressors and genes involved in OC metastasis such as integrins.Citation2,Citation3 Although HMT inhibitors (HMTI) that target EZH2 specifically or HMTs in general are promising anticancer therapeutics,Citation1 the mechanism(s) underlying EZH2 regulation is incompletely understood, and the use of clinically relevant model systems to better understand the translational potential of this important class of epigenetic modifiers is clearly an important area of investigation.
In the July 1, 2013 issue of Cell Cycle, Amatangelo and colleagues investigated the effect of a specific inhibitor of EZH2, GSK343, on human epithelial ovarian cancer.Citation4 After GSK343 treatment of ovarian cancer (OC) cells grown on a 2D monolayer, a 90% decrease in total H3K27me3 was observed, demonstrating drug specificity to EZH2, and EZH2 protein levels remained unchanged, corroborating the mechanism of GSK343 action as an inhibitor of EZH2 HMT activity. Despite this, no effect on growth was observed on OC cells in 2D culture, although previous studies have reported that reducing the HMT activity of EZH2 markedly alters cell physiology in OC and other cancers. For example, phosphorylation of EZH2 by protein kinase B (AKT) on serine 21 (S21) reduced EZH2 enzymatic activity and as well as integrin α2 expression.Citation5 In addition, EZH2 phosphorylation on residues threonine 350 and 487 (T350 and T487) by cyclin-dependent kinase 1 and 2 (CDK1 and CDK2), respectively, inhibited cell proliferation, migration, and anchorage-independent growth, indicating a role for EZH2 HMT activity in migration and invasion.Citation6,Citation7
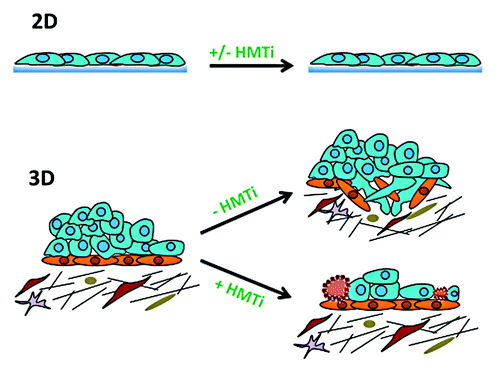
To further investigate the effect of GSK343-mediated EZH2 inhibition on OC, Amatangelo and colleaguesCitation4 extended the scope of their study by using a 3D cell culture system composed of a matrigel extracellular matrix (ECM). It is well known that the tumor microenvironment is both heterogeneous in nature, composed stromal fibroblasts, immune cells, and vascular endothelial cells, and can significantly impact both the metastatic potential of cancer cells as well as their ability to resist chemotherapy. For these reasons, the ECM closely recapitulates the tumor microenvironment, representing a more clinically relevant model system compared with monolayer culture conditions on plastic. Taking this approach, Amatangelo and colleagues observed that GSK343 treatment inhibited OC cell growth and invasion in a 3D culture and correlated with apoptosis induction in OC cells.Citation4 The results indicate regulation of EZH2 by the OC-ECM interaction as well as improved efficacy of GSK343 to suppress the ability of OC to remodel the ECM and metastasize ().
Figure 1. Comparing the sensitivity of epithelial ovarian cancer cells to the histone methyltransferase inhibitor (HMTI) GSK343 in 2D (i.e., plastic, upper) and 3D (i.e., matrigel extracellular matrix, lower). No effect of GSK343 on ovarian cancer cell migration, invasion, or apoptosis was observed using 2D culture conditions, despite the fact that the drug significantly reduced H3K27me3 levels. In 3D culture, however, GSK343 significantly inhibited ovarian cancer cell invasion and reduced cell survival, which was correlated with apoptosis induction (indicated by brown cells). The third dimension showcased the association of altered pathways (possibly by dysregulation of cellular proteins involved in ECM communication) with decreased in EZH2 activity.
As epigenetic therapies for OC are in the clinical arenaCitation8 (and see SGI-110 in Combination With Carboplatin in Ovarian Identifier: NCT01696032, CancerClinicalTrials.gov), the exciting new study by the Zhang lab provides compelling evidence for using 3D culture systems to gain key insight into the biological roles of EHZ2 in OC and the sensitivity of this cancer to EZH2-specific inhibitors. Furthermore, as suggested by the Zhang lab,Citation4 the numerous discrepancies observed between the efficacy of inhibitors in 2D culture and in vivo animal systems may be better explained by using the third dimension.
References
- McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 2012; 492:108 - 12; http://dx.doi.org/10.1038/nature11606; PMID: 23051747
- Li H, Cai Q, Godwin AK, Zhang R. Enhancer of zeste homolog 2 (EZH2) promotes the proliferation and invasion of epithelial ovarian cancer cells. Mol Cancer Res 2010; 12:1610 - 8; http://dx.doi.org/10.1158/1541-7786.MCR-10-0398
- Hu S, Yu L, Li Z, Shen Y, Wang J, Cai J, et al. Overexpression of EZH2 contributes to acquired cisplatin resistance in ovarian cancer cells in vitro and in vivo. Cancer Biol Ther 2010; 10:788 - 95; http://dx.doi.org/10.4161/cbt.10.8.12913; PMID: 20686362
- Amatangelo MD, Garipov A, Li H, Conejo-Garcia JR, Speicher DW, Zhang R. Three-dimensional culture sensitizes epithelial ovarian cancer cells to EZH2 methyltransferase inhibition. Cell Cycle 2013; 12; PMID: 23759589
- Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, et al. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science 2005; 310:306 - 10; http://dx.doi.org/10.1126/science.1118947; PMID: 16224021
- Ferraro A, Mourtzoukou D, Kosmidou V, Avlonitis S, Kontogeorgos G, Zografos G, et al. EZH2 is regulated by ERK/AKT and targets integrin alpha2 gene to control Epithelial-Mesenchymal Transition and anoikis in colon cancer cells. Int J Biochem Cell Biol 2013; 45:243 - 54; http://dx.doi.org/10.1016/j.biocel.2012.10.009; PMID: 23116973
- Chen S, Bohrer LR, Rai AN, Pan Y, Gan L, Zhou X, et al. Cyclin-dependent kinases regulate epigenetic gene silencing through phosphorylation of EZH2. Nat Cell Biol 2010; 12:1108 - 14; http://dx.doi.org/10.1038/ncb2116; PMID: 20935635
- Matei D, Fang F, Shen C, Schilder J, Arnold A, Zeng Y, et al. Epigenetic resensitization to platinum in ovarian cancer. Cancer Res 2012; 72:2197 - 205; http://dx.doi.org/10.1158/0008-5472.CAN-11-3909; PMID: 22549947