Abstract
Gonocytes (or prospermatogonia) are the precursors to spermatogonial stem cells (SSCs), which provide the foundation for spermatogenesis through their ability to both self-renew and generate daughter cells. Despite their relative importance, the regulatory mechanisms that govern gonocyte maintenance and transition to SSCs are poorly understood. Recently, we reported that constitutive activation of NOTCH1 signaling in Sertoli cells causes gonocyte exit from quiescence—the first suggestion of the potential role of this signaling pathway in the testis.
This Extra View will review what is known about NOTCH signaling, particularly in Sertoli cells and germ cells in the testes, by providing a background on germ cell biology and a summary of our recently published data on NOTCH1 signaling in Sertoli cells. We also describe additional data showing that aberrant proliferation and differentiation of gonocytes in response to constitutive activation of NOTCH1 signaling in Sertoli cells involves de novo expression of cell cycle proteins and a marked upregulation of the KIT receptor. These data further suggest that NOTCH signaling orchestrates a dynamic balance between maintenance and differentiation of gonocytes in the perinatal testis.
Introduction
A significant percentage of young men are infertile owing to a severe or complete lack of germ cells, and for the majority of these men, the underlying causes of the germ cell deficit remain unknown.Citation1-Citation3 In addition, the most common cancers affecting young men are testicular cancers of germ cell origin.Citation4 Despite recent progress, many aspects of mammalian germ cell proliferation and differentiation and the relationship between germ cells and their somatic niche are not understood. Sertoli cells provide for the germ cell microenvironment within the developing and adult testis by delivering growth factors and other signaling molecules that coordinate the precise development of germ cells throughout life. One such signal, previously identified in the testis of Caenorhabditis elegans, is the protein LAG-2, which is produced by the distal tip cell (a somatic cell equivalent to the vertebrate Sertoli cell), which forms the germline stem cell niche in C. elegans. LAG-2 associates with the GLP-1/NOTCH receptor at the surface of germ cells, and this signal maintains the germ cells in an undifferentiated mitotic state.Citation5 Lack of interaction between the distal tip cell and germ cells induces germ cell differentiation, meiosis, and gametogenesis. Therefore, in C. elegans, NOTCH signaling directly controls germline stem cell development and maintenance. In Drosophila, male germline stem cell maintenance depends not on NOTCH signaling, but rather on Unpaired (Upd) signals delivered by a group of somatic cells, called the hub, that form the niche.Citation6 However, it has recently become apparent that in Drosophila, NOTCH signaling is crucial for niche cell specification.Citation7,Citation8 Because evolutionarily conserved intracellular signaling mechanisms (such as NOTCH) are often employed to regulate germline niche formation and germ cell development, NOTCH signaling might also be employed in mammalian Sertoli cells to specify the fate of germ cells during embryonic development and after birth.
Here, we review what is currently known about germ cell development and the NOTCH signaling pathway in the mammalian testis, particularly in Sertoli cells. Then, we summarize results previously obtained by our group on the constitutive activation of NOTCH1 signaling in Sertoli cells and present additional data on the mechanism underlying the germ cell dysregulation we observed in our model. We close with an overview of our current understanding of the mechanisms regulating prenatal germ cell development.
Gonocytes Set the Stage for Spermatogenesis
In mammals, gonocytes (or prospermatogonia) are the precursors to spermatogonial stem cells (SSCs), which provide the foundation for spermatogenesis by their ability to both self-renew and generate daughter cells, which differentiate through meiosis to generate spermatozoa. Defects in proper gonocyte/SSC maintenance, in spermatogonia differentiation, or in the progression of meiosis lead to Sertoli cell-only syndrome or maturation arrest. These disorders are the leading causes of azoospermia, which occurs in 1% of all menCitation3 and in 10–15% of infertile men.Citation1,Citation2
During the embryonic development of male mice, primordial germ cells (PGCs) migrate to the genital ridges and by approximately embryonic day (E)12.5 are sequestered within the newly formed testis cords.Citation9 Between E12.5 and E14.5, PGCs differentiate into gonocytes (also referred to as T1 prospermatogonia) and undergo mitotic arrest (quiescence) in an unsynchronized manner, exiting the cell cycle at the G1/G0 transition.Citation10-Citation12 In mice, only after birth and between postnatal day (P)3 and P10 do the germ cells re-enter the cell cycle and resume mitosis, whereby they either develop into SSCs by P6 or enter prophase I of meiosis at puberty by P7 to P8.Citation9,Citation10,Citation13 Humans share the same developmental program, except that the timing is considerably extended, lasting the first 12 y of life.Citation9,Citation14 During the first 2–3 y in boys, gonocytes transition into reserve and active adult dark and adult pale spermatogonia. By 5 y of age, these adult dark and adult pale spermatogonia begin to differentiate into type B spermatogonia, which by the 10th year represent only 10% of all spermatogonia.Citation9,Citation14 Despite recent progress, a considerable gap in knowledge remains in our understanding of the regulatory mechanisms that govern the entry into and exit from gonocyte quiescence, the transition of gonocytes into SSCs, and the self-renewal vs. differentiation fate of SSCs. By elucidating these fundamental developmental mechanisms, it will be possible to determine the testicular causes of azoospermia and to design novel effective treatments for men with infertility.
The NOTCH Signaling Pathway
The NOTCH signaling pathway is best understood as a ligand- and receptor-based signaling pathway between adjacent cells, whereby both the ligands (JAG1, JAG2, DLL1, DLL3, and DLL4 in mammals) and receptors (NOTCH1 to NOTCH4 in mammals) are single-pass transmembrane proteins anchored to their respective cell surfaces. Upon ligand binding to a receptor, the intracellular domain of the receptor (NOTCH intracellular domain [NICD]) is released from the plasma membrane and translocates into the nucleus, where together with mastermind-like protein (MAML), NICD converts recombining binding protein suppressor of hairless (RBPJ) from a transcriptional repressor to a transcriptional activator. This activity triggers the expression of NOTCH target genes, the most notable of which are the hairy/enhancer-of-split (HES) and HES-related with YRPW motif (HEY) families of transcription factors.Citation15,Citation16 In general, HES and HEY proteins inhibit the expression of other genes by forming complexes with co-repressors.Citation17,Citation18
Modulation of NOTCH Signaling Through Receptor Modifications
NOTCH receptor activity can be regulated at numerous levels, including at glycosylation and fucosylation of the extracellular domain.Citation19,Citation20 The NOTCH extracellular domain contains many tandem epidermal growth factor-like repeats, which can be modified with O-linked fucose, glucose, or N-acetylglucosamine, which, in turn, modulates the affinity of the NOTCH receptors for their ligands.Citation19 The addition of O-fucose to epidermal growth factor-like repeats is catalyzed mainly by Protein O-fucosyltransferase 1 (POFUT1) in mammals,Citation21 and Lunatic fringe (LFNG), which belongs to a family of β1–3 N-acetyltransferases, can modify these O-fucose moieties.Citation22-Citation24 Pofut1 global null and Lfng global null mice display an embryonic lethal phenotype similar to mice lacking components of the NOTCH pathway,Citation25-Citation28 although presentation of this phenotype may be due to indirect effects.
Expression of NOTCH Signaling Components in Sertoli Cells and Germ Cells
The expression of NOTCH signaling components in neonatal and adult rodent and human testes was first reported in 2001.Citation29 Through co-culture of 6-d-old BALB/c mouse Sertoli cells and germ cells and subsequent immunocytochemical analyses, Dirami et al. demonstrated that 3 NOTCH receptors (NOTCH1, NOTCH2, and NOTCH3) and 2 NOTCH ligands (JAG1 and DLL1) were expressed on the cell surface of spermatogonia.Citation29 Sertoli cells were shown to express both ligands but only 1 of the 3 NOTCH receptors tested, NOTCH2.Citation29 Concurrently, the studies of Hayashi et al. demonstrated NOTCH1 and JAG2 immunostaining of rat and human spermatocytes as well as round and elongated spermatids in paraffin-embedded testes cross-sections.Citation30 Interestingly, the authors’ examination of NOTCH1 and JAG2 testis expression in 11 patients with maturation arrest revealed a complete lack of NOTCH2 expression, despite continued expression of JAG2.Citation30 In 2003, Mori et al. further characterized immunostaining of NOTCH1 to NOTCH4 in frozen sections of adult mouse testes and included the activated intracellular domains of the receptors in their analyses.Citation31 Their immunostaining demonstrated membrane-localized NOTCH1, NOTCH2, and NOTCH4 in spermatogonia, spermatocytes, and spermatids.Citation31 They also found nuclear-localized intracellular domain of NOTCH3 (NICD3) in spermatogonia and nuclear-localized NICD1 and NICD4 in spermatocytes and spermatids, indicating that the respective receptors had been activated in these cells.Citation31 Additionally, Sahin et al. demonstrated NOTCH2 immunostaining in spermatogonia and spermatocytes and NOTCH1 immunostaining in elongated spermatids.Citation32 Although the results of these studies generally disagree with regard to the particular receptor or ligand expressed in each cell type in the seminiferous epithelium, cumulatively, these studies strongly indicate that Sertoli cells and germ cells can elicit and respond to NOTCH signaling during spermatogenesis, and that the NOTCH signaling system may play a role in male germ cell differentiation and survival. However, these studies lack null mouse models of NOTCH signaling components linking NOTCH signaling in these cells with significant physiologic effects.
Reproductive Phenotypes of Mouse Models of NOTCH Signaling
Notch3−/−, Notch4−/−, Hes5−/−, Hey1−/−, and Heyl−/− global null mice are viable and fertile with no apparent lack of reproductive function.Citation15,Citation33-Citation36 Oppositely, Notch1−/−, Notch2−/−, Jag1−/−, Jag2−/−, Dll1−/−, Dll3−/−, Dll4-/+, Hes1−/−, Hes7−/−, and Hey2−/− mice cannot be studied for male reproductive function, because they all exhibit either complete embryonic or complete perinatal/postnatal lethal phenotype.Citation37-Citation47 Thus, using the Cre/loxP systemCitation48 and examining cell-specific null mouse models are of paramount importance for understanding the function and role of NOTCH signaling during spermatogenesis. Germ cell- and/or Sertoli cell-specific null mice of Notch1 that display no apparent phenotype have been generated,Citation49,Citation50 but cell-specific deletion and testing of the remaining receptors, ligands, and/or Hes/Hey target genes that otherwise bear a lethal phenotype in global null mice have not been reported to date.
Since NOTCH receptor, ligand and target gene paralogs may have redundant functions when deleted in a cell-specific fashion, recent studies of Sertoli and germ cell-specific disruption of the NOTCH pathway have aimed to target modulators of the NOTCH receptor that are considered less redundant than individual receptors, ligands, or Hes/Hey target genes. One such modulator that has been tested and independently reported by 2 groups is POFUT1.Citation49,Citation50 Results from both studies demonstrated that the NOTCH modifications brought about by POFUT1 are dispensable for testis development and spermatogenesis.Citation49,Citation50 However, previous studies on POFUT1 demonstrate that the protein is not required for stable cell surface expression of NOTCH receptor in mammals;Citation51 that an unrelated ER glucosidases can substitute for POFUT1 in promoting NOTCH folding and function;Citation52 and that mammalian NOTCH receptors are capable of signaling in the absence of POFUT1 and O-fucose.Citation52 Therefore, the finding that POFUT1 in Sertoli cells is dispensable for testis development and spermatogenesis does not necessarily preclude the possibility that NOTCH signaling can still function, either in entirety or to a limited or different extent, in these cells in modulating or helping to maintain proper regulation of spermatogenesis.
Because expression of NOTCH signaling components in a cell does not demonstrate that the pathway is truly activated, our recent study used a transgenic mouse model that expressed green fluorescent protein (GFP) in cells that actively received a NOTCH signal.Citation53 We observed that the canonical NOTCH signaling pathway is active exclusively in Sertoli cells in the perinatal testis. To circumvent redundancy and compensation in loss-of-function models, we then examined a Sertoli cell-specific NOTCH gain-of-function model in which NICD1 was constitutively expressed following Cre-mediated recombination in Sertoli cells (formerly Amh-cre;RosaNICD/+, hereafter referred to as AMH-NICD1).Citation53
AMH-NICD1 Mouse Model
It is well established that the maintenance and regulation of male germ cells rely on cues from the somatic environment, but the pathways that regulate gonocyte entry into and exit from quiescence, the transition of gonocytes into SSCs, and the self-renewal vs. differentiation fate of SSCs remain largely unknown. We demonstrated that increased activation of NOTCH signaling in Sertoli cells from approximately E13.5 onward led to drastic changes in gonocyte fate, including exit from quiescence, premature differentiation, and migration. These changes led to gonocyte apoptosis and to a Sertoli cell-only phenotype by P2. To glean additional insight into the molecular signature of the gonocytes in our model and to clarify the role for NOTCH signaling in Sertoli cells, we performed gene expression analysis of whole gonads isolated from E14.5 and E17.5 fetuses.Citation53 We found that several transcripts were differentially regulated in a manner consistent with NOTCH signaling activation in Sertoli cells and with premature differentiation of germ cells. For instance, RBPJ target genes such as Hey1 and Heyl were upregulated in Sertoli cells, as expected; in germ cells, Nanos2, a gene that suppresses meiosis,Citation54 was downregulated, and genes that are expressed at the beginning of or during meiosis, such as Stra8 and Rec8,Citation55,Citation56 were all upregulated (GSE37073). Furthermore, in accord with the observation that germ cell differentiation had taken place, a significant decrease in Pou5f1 (a marker for undifferentiated spermatogonia)Citation57 and a significant increase in Sohlh2 (a marker for differentiating spermatogonia)Citation58 were also found in these mutant germ cells. Our molecular analyses demonstrated that these effects correlated with a downregulation of Sertoli cell-specific factors that are essential to maintaining the undifferentiated state of germ cells. These factors include glial cell-derived neurotrophic factor (GDNF), which is required for fetal germ cell proliferation and survivalCitation59,Citation60 and for spermatogonial stem cell self-renewal,Citation61,Citation62 and CYP26B1, an enzyme that inhibits germ cell progression toward meiosis.Citation63 Conversely, complete inhibition of NOTCH signaling in Sertoli cells in vitro by the NOTCH inhibitor DAPT resulted in the opposite phenotype, with dose-dependent increases in Gdnf and Cyp26b1 expression. These data demonstrated that NOTCH signaling in perinatal Sertoli cells maintains gonocyte quiescence by establishing the proper balance between germ cell proliferation and differentiation.
Molecular Signature of Germ Cells in Wild-Type and AMH-NICD1 Mouse Testes
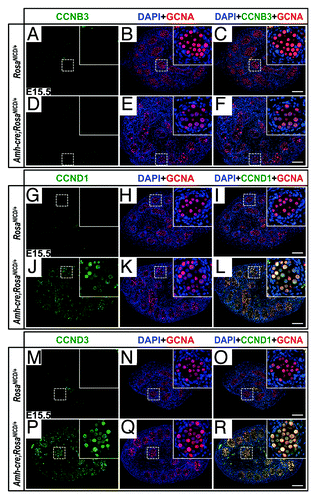
In male wild-type mice at E13.5, most PGCs have entered the bipotential gonads. By E15.5, they have all reached mitotic arrest and become gonocytes. Mitotic arrest coincides with downregulation of expression of CCND3 and CCNB1, the major cyclins found in PGCs.Citation64,Citation65 The gonocytes remain mitotically quiescent until shortly after birth, when cyclins are re-expressed and the cell cycle resumes. Germ cells then actively proliferate as they differentiate and express different types of cyclins depending on the stage of maturation.Citation66 Previous research demonstrated that CCND1 is expressed in spermatogonia only; that CCND2 and CCND3 are expressed throughout the premeiotic and meiotic stages; and that CCNB3 is uniquely expressed in leptotene and zygotene spermatocytes.Citation66 Therefore, the particular expression pattern of cyclins in the mutant germ cells may represent the cells’ level of differentiation.
According to our microarray and quantitative real-time polymerase chain reaction data analysis, at E14.5, Ccnd1 transcripts were significantly upregulated in AMH-NICD1 gonads, at levels 3 times those of the littermate controls, suggesting that the germ cells had actively proliferated; indeed, the mutant testes had significantly more germ cells than did the controls.Citation53 We further examined protein expression of CCND1, as well as CCNB3 and CCND3, to glean additional insight into the stage of differentiation of the aberrant gonocytes. Through immunohistochemistry, we found that CCND1 and CCND3, but not CCNB3, were markedly upregulated in mutant gonocytes at E15.5. (); in stark contrast, the control gonads showed no staining above background levels for any of the cyclins tested at this age (). Therefore, the aberrant gonocytes presented signs of differentiation toward spermatogonia but were not advanced enough to proceed through the first meiotic division.
Figure 1. Aberrant germ cell proliferation and differentiation in AMH-NICD1 testes involves CCND1 and CCND3, but not CCNB3. (A–R) Immunofluorescence images of control (RosaNICD/+) (A–C, G–I, and M–O) and AMH-NICD1 (Amh-cre;RosaNICD/+) (D–F, J–L, P–R) testis sections at E15.5, showing CCNB3 (A–F; green), CCND1 (G–L; green), CCND3 (M–R; green), germ cell nuclear antigen (GCNA; A–R; red), and DAPI (A–R; blue) staining. Scale bars represent 100 µm. Higher magnification inset in upper right of each image. Immunostainings are representative of at least 6 separate gonad cross-sections, from at least 3 separate fetuses.
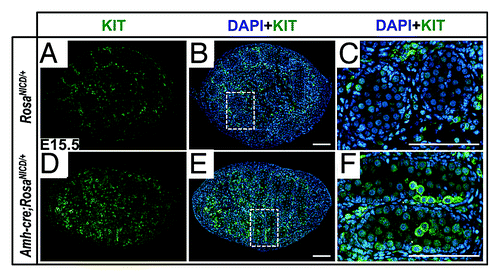
In mammals, the KIT membrane receptor at the surface of germ cells and the receptor’s ligand (KITL), produced by Sertoli cells, are of crucial importance for normal germ cell development in the embryo and after birth.Citation67 Additionally, KIT is a well-known marker for PGCs. Together with KITL, KIT mediates PGC proliferation and survival as well as PGC migration toward the genital ridges.Citation68 In wild-type mice, after the germ cells have reached the male gonads and are quiescent, KIT expression is downregulated. KIT is then re-expressed in a fraction of gonocytes after birth and is thought to mediate their migration toward the basement membrane.Citation69-Citation71 While spermatogonial stem cells and other undifferentiated spermatogonia do not express KIT, this molecule is expressed in all differentiating spermatogonia in the first wave of spermatogenesis and in the adult.Citation72 Our microarray data set (GSE37073) revealed that Kit transcripts were upregulated in AMH-NICD1 to levels 1.63 times those of the controls at E14.5 (p = 2.45 × 10−2). To determine whether KIT protein levels also were upregulated, we stained gonads at E15.5 with anti-KIT antibody. As shown in , KIT was indeed markedly upregulated in a sub-population of gonocytes in the mutant. Because KIT expression was upregulated concomitant with a decrease in Pou5f1 and an increase in Sohlh2, Stra8, Sycp3, and Rec8,Citation53 our data overall establish that the aberrant gonocytes had acquired properties of differentiating spermatogonia.
Figure 2. Germ cells in AMH-NICD1 testes express KIT at high levels in a subpopulation of germ cells. (A–F) Immunofluorescence images of control (RosaNICD/+) (A–C) and AMH-NICD1 (Amh-cre;RosaNICD/+) (D–F) testis sections at E15.5, showing KIT (green), germ cell nuclear antigen (GCNA; red), and DAPI (blue) staining. Scale bars represent 100 µm. Areas outlined in panels (B and E) are magnified 4 times in panels (C and F), rotated 90° clockwise and counter-clockwise, respectively. Immunostainings are representative of at least 6 separate gonad cross-sections, from at least 3 separate fetuses.
Phenotype of Sertoli cells in Wild-Type and AMH-NICD1 Testes
The gonadal microenvironment is crucial for the control of germ cell differentiation. After they have arrived in the male gonad, the germ cells are nurtured by the Sertoli cells, which form the major component of the germ cell microenvironment. Through the timely delivery of growth and differentiation factors, Sertoli cells coordinate the precise development of the germ cells throughout life. One such growth factor is GDNF, which is already expressed at high levels by precursors of Sertoli cells at E11.5,Citation59 and which binds to a receptor complex formed by the receptors RET and GFRA1 in fetal and adult germ cells.Citation60,Citation73,Citation74 Miles et al. recently investigated the putative function of GDNF signaling in the fetal gonad and demonstrated that Gndf is exclusively expressed in the male gonad between E12.5 and E15.5,Citation60 when gonocytes have entered the gonads but still proliferate until E15.5. These authors demonstrated that RET signaling is required for gonocyte proliferation and maintenance, and that Ret−/− germ cells underwent apoptosis.
As we previously reported, transcript levels for Gdnf were significantly downregulated in the AMH-NICD1 mutant testes at E14.5 and E17.5 and in cultures of Sertoli cells isolated from mutant testes at P10.Citation53 In addition, γ-secretase inhibitor DAPT-induced downregulation of NOTCH signaling in Sertoli cells in vitro upregulated Gdnf expression levels. These findings therefore strongly suggest that NOTCH signaling is a negative regulator of GDNF production that counteracts the positive effects of FGF2Citation75 (). NOTCH signaling specifically downregulates the levels of expression of GDNF in the perinatal testis and may contribute to the entry of gonocytes into mitotic arrest (M-prospermatogonia into T1-prospermatogonia). Since the levels of GDNF increase after birth and influence gonocyte migration (T2-prospermatogonia)Citation76 and spermatogonial stem cell self-renewal,Citation61,Citation62 GDNF expression in the perinatal testis must be cyclic and may be inversely related to the cyclicity of NOTCH activity.Citation49 NOTCH signaling may maintain gonocytes in a quiescent state by keeping GDNF at basal levels to ensure gonocyte survival; however, a lack of GDNF through NOTCH signaling overactivation induces germ cell apoptosis.
Figure 3. Model depicting the possible role of NOTCH signaling in Sertoli cells before birth. Previous studies have shown that FGF2 induces GDNF production, and that SOX9/SF1 induces CYP26B1 expression by Sertoli cells. Our data suggest NOTCH1 signaling is a negative regulator of GDNF and CYP26B1, which balances the effects of FGF2 and SOX9/SF1. This normally results in gonocyte maintenance in the quiescent, undifferentiated state. However, overactivation of NOTCH1 signaling further suppresses the expression of GDNF and CYP26B1, which induces direct differentiation into KIT-expressing proliferating spermatogonia.
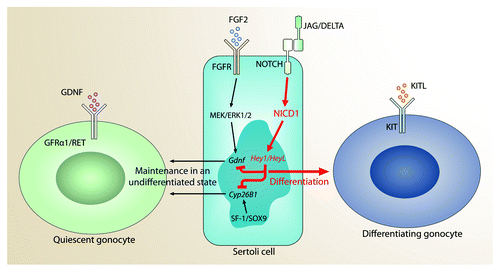
We also demonstrated that overactivation of NOTCH signaling in vivo downregulated CYP26B1, an enzyme crucial for maintaining germ cells in an undifferentiated state.Citation77,Citation78 Abrogation of NOTCH activation by DAPT in Sertoli cells in vitro resulted in an increase in Cyp26b1 expression, indicating that NOTCH signaling downregulates the levels of this enzyme. Normally, CYP26B1 in Sertoli and Leydig cells regulates the concentration of retinoic acid by catabolizing all-trans-retinoic acid into inactive oxidized metabolites.Citation79-Citation81 Since retinoic acid controls germ cell progression toward meiosis, the presence of CYP26B1 in the male embryo and perinatal testis prevents meiotic entry.Citation82 Therefore, decrease of CYP26B1 in the AMH-NICD1 gonads likely promotes both premature differentiation of gonocytes into spermatogonia and germ cell progression toward meiosis, causing the cells to express Kit, Stra8, and Sycp3, as reported in our previous investigation.Citation53 Until the recent study by Kashimada et al., little was known about the regulation of CYP26B1 expression in normal gonads.Citation83 It now appears that SOX9 and SF1 significantly activate Cyp26b1 expression in vivo in male mice,Citation83 which represses meiosis until puberty. Therefore, NOTCH appears to promote differentiation toward meiosis by negatively regulating Cyp26b1. However, the effects of SOX9 and SF1 presumably outweigh the effects of NOTCH in fetal Sertoli cells (). Sex reversal was not observed in the AMH-NICD1 model, because the levels of Sox9,Citation84,Citation85 Dax1,Citation86 and Foxl2Citation87 expression in Sertoli cells were not significantly altered in our microarray (GSE37073).
Conclusion
The NOTCH signaling pathway is crucial for tissue patterning and homeostasis and plays an important role in regulating C. elegans spermatogenesis and the Drosophila male germ cell niche.Citation5,Citation7,Citation8 In mice, this pathway’s role in the development of the ovaries and oocytes has begun to be clarified;Citation88,Citation89 however, its role in controlling the fate of gonocytes and the development of male germ cells after birth and into adulthood is still not clear. We have now demonstrated that NOTCH signaling in Sertoli cells is a crucial modulator of germ cell development, and that when overactivated, NOTCH signaling inhibits quiescence and pushes germ cells toward differentiation and meiosis (). While further research is required to decipher the molecular pathways underlying these effects, as well as the relationship between NOTCH and other signaling pathways in Sertoli cells and germ cells, it appears that the absence of NOTCH activation ensures germ cell proliferation, whereas NOTCH overactivation promotes germ cell differentiation toward meiosis and, ultimately, apoptosis. However, it is not known whether germ cell apoptosis is linked to inadequate levels of GDNF and CYP26B1 or to an inadequate response of the microenvironment to prematurely differentiating germ cells. In addition, data from our in vitro experiment suggest that loss of NOTCH signaling may induce a germ cell differentiation block by increasing the levels of GDNF produced by Sertoli cells. This event implies that dysregulation of this pathway plays a role in the maintenance and growth of testicular germ cell tumors, as previously suggested by others.Citation90
Materials and Methods
Generation of conditional AMH-NICD1 mice
Heterozygous Tg(AMH-cre)1Flor (Amh-cre)Citation91 female mice were mated to homozygous floxed Gt(Rosa)26Sortm1(Notch1)Dam/J (RosaNICD)Citation92 male mice to generate F1 Amh-cre;RosaNICD/+ overexpressors and RosaNICD/+ controls as previously described.Citation53
Histology and immunohistochemistry
Immunohistochemistry of RosaNICD and Amh-cre;RosaNICD testes was performed as previously described.Citation53 All multi-channel fluorescent images shown in the figures and used for analysis were tiled and stitched images of entire gonad cross-sections, acquired with a Nikon A1 laser scanning confocal microscope (Nikon, Tokyo, Japan) equipped with the NIS-Elements acquisition and analysis software platform (version 4.1), with equivalent exposure levels between control and mutant sections at each staining. The primary antibodies used and their dilutions are as follows: CCNB3 (Santa Cruz Biotechnology; Cat. No. SC-131482; 1:1000), CCND1 (Abcam; Cat. No. AB6152; 1:1000), CCND3 (Abcam; Cat. No. AB28283; 1:1000), germ cell nuclear antigen (GCNA; a generous gift of Dr George Enders;Citation93 1:30), and KIT (R&D Systems, Minneapolis, MN; Cat. No. AF1356; 1:40). Each staining was performed with at least 6 separate testes from at least 3 separate fetuses.
| Abbreviations: | ||
| SSCs | = | spermatogonial stem cells |
| PGCs | = | primordial germ cells |
| E | = | embryonic day |
| P | = | postnatal day |
| NICD | = | NOTCH intracellular domain |
Acknowledgments
This work was supported by NIH R01 HD044543 and R21 HD068989 to MCH and in part through Cancer Center Support Grant CA16672. We are also grateful to Dr Walter N Hittelman and the Center for Targeted Therapy at The University of Texas MD Anderson Cancer Center for generously providing access to the Nikon confocal microscope.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Male Reproduction and Urology. Evaluation of the azoospermic male. Fertil Steril 2008; 90:Suppl S74 - 7; http://dx.doi.org/10.1016/j.fertnstert.2008.08.092; PMID: 19007652
- Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol 1989; 142:62 - 5; PMID: 2499695
- Stephen EH, Chandra A. Declining estimates of infertility in the United States: 1982-2002. Fertil Steril 2006; 86:516 - 23; http://dx.doi.org/10.1016/j.fertnstert.2006.02.129; PMID: 16952500
- Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update 2006; 12:303 - 23; http://dx.doi.org/10.1093/humupd/dmk006; PMID: 16540528
- Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol 2007; 23:405 - 33; http://dx.doi.org/10.1146/annurev.cellbio.23.090506.123326; PMID: 17506698
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 2001; 294:2542 - 5; http://dx.doi.org/10.1126/science.1066707; PMID: 11752574
- Kitadate Y, Kobayashi S. Notch and Egfr signaling act antagonistically to regulate germ-line stem cell niche formation in Drosophila male embryonic gonads. Proc Natl Acad Sci U S A 2010; 107:14241 - 6; http://dx.doi.org/10.1073/pnas.1003462107; PMID: 20660750
- Okegbe TC, DiNardo S. The endoderm specifies the mesodermal niche for the germline in Drosophila via Delta-Notch signaling. Development 2011; 138:1259 - 67; http://dx.doi.org/10.1242/dev.056994; PMID: 21350008
- Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C Embryo Today 2009; 87:1 - 26; http://dx.doi.org/10.1002/bdrc.20142; PMID: 19306346
- Hilscher B, Hilscher W, Bülthoff-Ohnolz B, Krämer U, Birke A, Pelzer H, et al. Kinetics of gametogenesis. I. Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell Tissue Res 1974; 154:443 - 70; PMID: 4442109
- McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol 1997; 187:107 - 13; http://dx.doi.org/10.1006/dbio.1997.8584; PMID: 9224678
- Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH. Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells 2008; 26:339 - 47; http://dx.doi.org/10.1634/stemcells.2007-0622; PMID: 18024419
- Bellvé AR, Cavicchia JC, Millette CF, O’Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol 1977; 74:68 - 85; http://dx.doi.org/10.1083/jcb.74.1.68; PMID: 874003
- Paniagua R, Nistal M. Morphological and histometric study of human spermatogonia from birth to the onset of puberty. J Anat 1984; 139:535 - 52; PMID: 6490534
- Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J 1999; 18:2196 - 207; http://dx.doi.org/10.1093/emboj/18.8.2196; PMID: 10205173
- Iso T, Sartorelli V, Chung G, Shichinohe T, Kedes L, Hamamori Y. HERP, a new primary target of Notch regulated by ligand binding. Mol Cell Biol 2001; 21:6071 - 9; http://dx.doi.org/10.1128/MCB.21.17.6071-6079.2001; PMID: 11486044
- Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 2003; 194:237 - 55; http://dx.doi.org/10.1002/jcp.10208; PMID: 12548545
- Fischer A, Gessler M. Delta-Notch--and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res 2007; 35:4583 - 96; http://dx.doi.org/10.1093/nar/gkm477; PMID: 17586813
- Takeuchi H, Haltiwanger RS. Role of glycosylation of Notch in development. Semin Cell Dev Biol 2010; 21:638 - 45; http://dx.doi.org/10.1016/j.semcdb.2010.03.003; PMID: 20226260
- Rana NA, Haltiwanger RS. Fringe benefits: functional and structural impacts of O-glycosylation on the extracellular domain of Notch receptors. Curr Opin Struct Biol 2011; 21:583 - 9; http://dx.doi.org/10.1016/j.sbi.2011.08.008; PMID: 21924891
- Wang Y, Shao L, Shi S, Harris RJ, Spellman MW, Stanley P, et al. Modification of epidermal growth factor-like repeats with O-fucose. Molecular cloning and expression of a novel GDP-fucose protein O-fucosyltransferase. J Biol Chem 2001; 276:40338 - 45; PMID: 11524432
- Johnston SH, Rauskolb C, Wilson R, Prabhakaran B, Irvine KD, Vogt TF. A family of mammalian Fringe genes implicated in boundary determination and the Notch pathway. Development 1997; 124:2245 - 54; PMID: 9187150
- Brückner K, Perez L, Clausen H, Cohen S. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature 2000; 406:411 - 5; http://dx.doi.org/10.1038/35019075; PMID: 10935637
- Shimizu K, Chiba S, Saito T, Kumano K, Takahashi T, Hirai H. Manic fringe and lunatic fringe modify different sites of the Notch2 extracellular region, resulting in different signaling modulation. J Biol Chem 2001; 276:25753 - 8; http://dx.doi.org/10.1074/jbc.M103473200; PMID: 11346656
- Evrard YA, Lun Y, Aulehla A, Gan L, Johnson RL. lunatic fringe is an essential mediator of somite segmentation and patterning. Nature 1998; 394:377 - 81; http://dx.doi.org/10.1038/28632; PMID: 9690473
- Zhang N, Gridley T. Defects in somite formation in lunatic fringe-deficient mice. Nature 1998; 394:374 - 7; http://dx.doi.org/10.1038/28625; PMID: 9690472
- Okajima T, Irvine KD. Regulation of notch signaling by o-linked fucose. Cell 2002; 111:893 - 904; http://dx.doi.org/10.1016/S0092-8674(02)01114-5; PMID: 12526814
- Shi S, Stanley P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc Natl Acad Sci U S A 2003; 100:5234 - 9; http://dx.doi.org/10.1073/pnas.0831126100; PMID: 12697902
- Dirami G, Ravindranath N, Achi MV, Dym M. Expression of Notch pathway components in spermatogonia and Sertoli cells of neonatal mice. J Androl 2001; 22:944 - 52; PMID: 11700858
- Hayashi T, Kageyama Y, Ishizaka K, Xia G, Kihara K, Oshima H. Requirement of Notch 1 and its ligand jagged 2 expressions for spermatogenesis in rat and human testes. J Androl 2001; 22:999 - 1011; PMID: 11700865
- Mori S, Kadokawa Y, Hoshinaga K, Marunouchi T. Sequential activation of Notch family receptors during mouse spermatogenesis. Dev Growth Differ 2003; 45:7 - 13; http://dx.doi.org/10.1046/j.1440-169X.2003.00670.x; PMID: 12630942
- Sahin Z, Bayram Z, Celik-Ozenci C, Akkoyunlu G, Seval Y, Erdogru T, et al. Effect of experimental varicocele on the expressions of Notch 1, 2, and 3 in rat testes: an immunohistochemical study. Fertil Steril 2005; 83:86 - 94; http://dx.doi.org/10.1016/j.fertnstert.2004.09.006; PMID: 15652892
- Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev 2004; 18:901 - 11; http://dx.doi.org/10.1101/gad.291004; PMID: 15107403
- Fischer A, Steidl C, Wagner TU, Lang E, Jakob PM, Friedl P, et al. Combined loss of Hey1 and HeyL causes congenital heart defects because of impaired epithelial to mesenchymal transition. Circ Res 2007; 100:856 - 63; http://dx.doi.org/10.1161/01.RES.0000260913.95642.3b; PMID: 17303760
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 2000; 14:1343 - 52; PMID: 10837027
- Krebs LT, Xue Y, Norton CR, Sundberg JP, Beatus P, Lendahl U, et al. Characterization of Notch3-deficient mice: normal embryonic development and absence of genetic interactions with a Notch1 mutation. Genesis 2003; 37:139 - 43; http://dx.doi.org/10.1002/gene.10241; PMID: 14595837
- Bessho Y, Sakata R, Komatsu S, Shiota K, Yamada S, Kageyama R. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev 2001; 15:2642 - 7; http://dx.doi.org/10.1101/gad.930601; PMID: 11641270
- Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development 1995; 121:1533 - 45; PMID: 7789282
- Dunwoodie SL, Clements M, Sparrow DB, Sa X, Conlon RA, Beddington RS. Axial skeletal defects caused by mutation in the spondylocostal dysplasia/pudgy gene Dll3 are associated with disruption of the segmentation clock within the presomitic mesoderm. Development 2002; 129:1795 - 806; PMID: 11923214
- Gessler M, Knobeloch KP, Helisch A, Amann K, Schumacher N, Rohde E, et al. Mouse gridlock: no aortic coarctation or deficiency, but fatal cardiac defects in Hey2 -/- mice. Curr Biol 2002; 12:1601 - 4; http://dx.doi.org/10.1016/S0960-9822(02)01150-8; PMID: 12372253
- Hamada Y, Kadokawa Y, Okabe M, Ikawa M, Coleman JR, Tsujimoto Y. Mutation in ankyrin repeats of the mouse Notch2 gene induces early embryonic lethality. Development 1999; 126:3415 - 24; PMID: 10393120
- Hrabĕ de Angelis M, McIntyre J 2nd, Gossler A. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature 1997; 386:717 - 21; http://dx.doi.org/10.1038/386717a0; PMID: 9109488
- Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev 1995; 9:3136 - 48; http://dx.doi.org/10.1101/gad.9.24.3136; PMID: 8543157
- Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV, et al. Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev 1998; 12:1046 - 57; http://dx.doi.org/10.1101/gad.12.7.1046; PMID: 9531541
- Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev 2004; 18:2469 - 73; http://dx.doi.org/10.1101/gad.1239204; PMID: 15466160
- Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T. Notch1 is essential for postimplantation development in mice. Genes Dev 1994; 8:707 - 19; http://dx.doi.org/10.1101/gad.8.6.707; PMID: 7926761
- Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, et al. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet 1999; 8:723 - 30; http://dx.doi.org/10.1093/hmg/8.5.723; PMID: 10196361
- Orban PC, Chui D, Marth JD. Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci U S A 1992; 89:6861 - 5; http://dx.doi.org/10.1073/pnas.89.15.6861; PMID: 1495975
- Hasegawa K, Okamura Y, Saga Y. Notch signaling in Sertoli cells regulates cyclical gene expression of Hes1 but is dispensable for mouse spermatogenesis. Mol Cell Biol 2012; 32:206 - 15; PMID: 22037762
- Batista F, Lu L, Williams SA, Stanley P. Complex N-glycans are essential, but core 1 and 2 mucin O-glycans, O-fucose glycans, and NOTCH1 are dispensable, for mammalian spermatogenesis. Biol Reprod 2012; 86:179; http://dx.doi.org/10.1095/biolreprod.111.098103; PMID: 22492969
- Yao D, Huang Y, Huang X, Wang W, Yan Q, Wei L, et al. Protein O-fucosyltransferase 1 (Pofut1) regulates lymphoid and myeloid homeostasis through modulation of Notch receptor ligand interactions. Blood 2011; 117:5652 - 62; http://dx.doi.org/10.1182/blood-2010-12-326074; PMID: 21464368
- Stahl M, Uemura K, Ge C, Shi S, Tashima Y, Stanley P. Roles of Pofut1 and O-fucose in mammalian Notch signaling. J Biol Chem 2008; 283:13638 - 51; http://dx.doi.org/10.1074/jbc.M802027200; PMID: 18347015
- Garcia TX, DeFalco T, Capel B, Hofmann MC. Constitutive activation of NOTCH1 signaling in Sertoli cells causes gonocyte exit from quiescence. Dev Biol 2013; 377:188 - 201; http://dx.doi.org/10.1016/j.ydbio.2013.01.031; PMID: 23391689
- Barrios F, Filipponi D, Pellegrini M, Paronetto MP, Di Siena S, Geremia R, et al. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci 2010; 123:871 - 80; http://dx.doi.org/10.1242/jcs.057968; PMID: 20159962
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, et al. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A 2008; 105:14976 - 80; http://dx.doi.org/10.1073/pnas.0807297105; PMID: 18799751
- Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC. Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis 2004; 40:184 - 94; http://dx.doi.org/10.1002/gene.20085; PMID: 15515002
- Pesce M, Wang X, Wolgemuth DJ, Schöler H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev 1998; 71:89 - 98; http://dx.doi.org/10.1016/S0925-4773(98)00002-1; PMID: 9507072
- Suzuki H, Ahn HW, Chu T, Bowden W, Gassei K, Orwig K, et al. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Dev Biol 2012; 361:301 - 12; PMID: 22056784
- Beverdam A, Koopman P. Expression profiling of purified mouse gonadal somatic cells during the critical time window of sex determination reveals novel candidate genes for human sexual dysgenesis syndromes. Hum Mol Genet 2006; 15:417 - 31; http://dx.doi.org/10.1093/hmg/ddi463; PMID: 16399799
- Miles DC, van den Bergen JA, Wakeling SI, Anderson RB, Sinclair AH, Western PS. The proto-oncogene Ret is required for male foetal germ cell survival. Dev Biol 2012; 365:101 - 9; http://dx.doi.org/10.1016/j.ydbio.2012.02.014; PMID: 22360967
- Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000; 287:1489 - 93; http://dx.doi.org/10.1126/science.287.5457.1489; PMID: 10688798
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 2004; 101:16489 - 94; http://dx.doi.org/10.1073/pnas.0407063101; PMID: 15520394
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, et al. Retinoid signaling determines germ cell fate in mice. Science 2006; 312:596 - 600; http://dx.doi.org/10.1126/science.1125691; PMID: 16574820
- Sorrentino E, Nazzicone V, Farini D, Campagnolo L, De Felici M. Comparative transcript profiles of cell cycle-related genes in mouse primordial germ cells, embryonic stem cells and embryonic germ cells. Gene Expr Patterns 2007; 7:714 - 21; http://dx.doi.org/10.1016/j.modgep.2007.02.002; PMID: 17398164
- Spiller CM, Koopman P. Cell cycle control of germ cell differentiation. Results Probl Cell Differ 2011; 53:269 - 308; http://dx.doi.org/10.1007/978-3-642-19065-0_13; PMID: 21630150
- Wolgemuth DJ, Roberts SS. Regulating mitosis and meiosis in the male germ line: critical functions for cyclins. Philos Trans R Soc Lond B Biol Sci 2010; 365:1653 - 62; http://dx.doi.org/10.1098/rstb.2009.0254; PMID: 20403876
- Besmer P, Manova K, Duttlinger R, Huang EJ, Packer A, Gyssler C, et al. The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev Suppl 1993; •••:125 - 37; PMID: 7519481
- Farini D, La Sala G, Tedesco M, De Felici M. Chemoattractant action and molecular signaling pathways of Kit ligand on mouse primordial germ cells. Dev Biol 2007; 306:572 - 83; http://dx.doi.org/10.1016/j.ydbio.2007.03.031; PMID: 17467686
- Ohbo K, Yoshida S, Ohmura M, Ohneda O, Ogawa T, Tsuchiya H, et al. Identification and characterization of stem cells in prepubertal spermatogenesis in mice small star, filled. Dev Biol 2003; 258:209 - 25; http://dx.doi.org/10.1016/S0012-1606(03)00111-8; PMID: 12781694
- Orth JM, Jester WF Jr., Qiu J. Gonocytes in testes of neonatal rats express the c-kit gene. Mol Reprod Dev 1996; 45:123 - 31; http://dx.doi.org/10.1002/(SICI)1098-2795(199610)45:2<123::AID-MRD3>3.0.CO;2-V; PMID: 8914068
- Orth JM, Qiu J, Jester WF Jr., Pilder S. Expression of the c-kit gene is critical for migration of neonatal rat gonocytes in vitro. Biol Reprod 1997; 57:676 - 83; http://dx.doi.org/10.1095/biolreprod57.3.676; PMID: 9283007
- Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology 1999; 140:5894 - 900; http://dx.doi.org/10.1210/en.140.12.5894; PMID: 10579355
- Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol 2005; 279:114 - 24; http://dx.doi.org/10.1016/j.ydbio.2004.12.006; PMID: 15708562
- Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol 2008; 288:95 - 103; http://dx.doi.org/10.1016/j.mce.2008.04.012; PMID: 18485583
- Simon L, Ekman GC, Tyagi G, Hess RA, Murphy KM, Cooke PS. Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp Cell Res 2007; 313:3090 - 9; http://dx.doi.org/10.1016/j.yexcr.2007.05.002; PMID: 17574550
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol 2005; 6:314 - 22; http://dx.doi.org/10.1038/ni1164; PMID: 15665828
- Li H, MacLean G, Cameron D, Clagett-Dame M, Petkovich M. Cyp26b1 expression in murine Sertoli cells is required to maintain male germ cells in an undifferentiated state during embryogenesis. PLoS One 2009; 4:e7501; http://dx.doi.org/10.1371/journal.pone.0007501; PMID: 19838304
- MacLean G, Li H, Metzger D, Chambon P, Petkovich M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 2007; 148:4560 - 7; http://dx.doi.org/10.1210/en.2007-0492; PMID: 17584971
- Fujii H, Sato T, Kaneko S, Gotoh O, Fujii-Kuriyama Y, Osawa K, et al. Metabolic inactivation of retinoic acid by a novel P450 differentially expressed in developing mouse embryos. EMBO J 1997; 16:4163 - 73; http://dx.doi.org/10.1093/emboj/16.14.4163; PMID: 9250660
- White JA, Beckett-Jones B, Guo YD, Dilworth FJ, Bonasoro J, Jones G, et al. cDNA cloning of human retinoic acid-metabolizing enzyme (hP450RAI) identifies a novel family of cytochromes P450. J Biol Chem 1997; 272:18538 - 41; http://dx.doi.org/10.1074/jbc.272.30.18538; PMID: 9228017
- White JA, Guo YD, Baetz K, Beckett-Jones B, Bonasoro J, Hsu KE, et al. Identification of the retinoic acid-inducible all-trans-retinoic acid 4-hydroxylase. J Biol Chem 1996; 271:29922 - 7; http://dx.doi.org/10.1074/jbc.271.47.29922; PMID: 8939936
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 2006; 103:2474 - 9; http://dx.doi.org/10.1073/pnas.0510813103; PMID: 16461896
- Kashimada K, Svingen T, Feng CW, Pelosi E, Bagheri-Fam S, Harley VR, et al. Antagonistic regulation of Cyp26b1 by transcription factors SOX9/SF1 and FOXL2 during gonadal development in mice. FASEB J 2011; 25:3561 - 9; http://dx.doi.org/10.1096/fj.11-184333; PMID: 21757499
- Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet 1996; 14:62 - 8; http://dx.doi.org/10.1038/ng0996-62; PMID: 8782821
- Sekido R, Bar I, Narváez V, Penny G, Lovell-Badge R. SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol 2004; 274:271 - 9; http://dx.doi.org/10.1016/j.ydbio.2004.07.011; PMID: 15385158
- Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL. Role of Ahch in gonadal development and gametogenesis. Nat Genet 1998; 20:353 - 7; http://dx.doi.org/10.1038/3822; PMID: 9843206
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 2009; 139:1130 - 42; http://dx.doi.org/10.1016/j.cell.2009.11.021; PMID: 20005806
- Trombly DJ, Woodruff TK, Mayo KE. Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology 2009; 150:1014 - 24; http://dx.doi.org/10.1210/en.2008-0213; PMID: 18818300
- Manosalva I, González A, Kageyama R. Hes1 in the somatic cells of the murine ovary is necessary for oocyte survival and maturation. Dev Biol 2013; 375:140 - 51; http://dx.doi.org/10.1016/j.ydbio.2012.12.015; PMID: 23274689
- Hayashi T, Yamada T, Kageyama Y, Kihara K. Expression failure of the notch signaling system is associated with the pathogenesis of testicular germ cell tumor. Tumour Biol 2004; 25:99 - 105; http://dx.doi.org/10.1159/000079140; PMID: 15361705
- Lécureuil C, Fontaine I, Crepieux P, Guillou F. Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis 2002; 33:114 - 8; http://dx.doi.org/10.1002/gene.10100; PMID: 12124943
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A 2003; 100:14920 - 5; http://dx.doi.org/10.1073/pnas.2436557100; PMID: 14657333
- Enders GC, May JJ 2nd. Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic day 11 to adult in male and female mice. Dev Biol 1994; 163:331 - 40; http://dx.doi.org/10.1006/dbio.1994.1152; PMID: 8200475