Hydrogen sulfide (H2S) is an endogenous gaseous biological mediator. In the cardiovascular system, it acts as a vasodilator, proangiogenic hormone, and cardioprotectant, while in the nervous system, it functions as a neurotransmitter and neuroprotectant.Citation1,Citation2 Our interest in the role of H2S in cancer biology was initially triggered by our observations in endothelial cells showing that the pro-angiogenic effect of VEGF (vascular endothelial growth factor, a principal tumor angiogenic hormone) is mediated in part by endogenous H2S.Citation3 VEGF increased H2S production in endothelial cells, and inhibition of CSE (cystathionine gamma-lyase, a major H2S-producing enzyme) attenuated the proangiogenic effects of VEGF in vitro.Citation3 Our original hypothesis was that cancer cell-derived VEGF stimulates tumor angiogenesis via stimulation of H2S production in peritumoral tissues.
While experimentally testing this hypothesis, we have discovered that colorectal cancer tissue from human surgical resections contains markedly elevated levels of a different H2S-producing enzyme, cystathionine-β-synthase (CBS).Citation4 Likewise, the colon cancer-derived epithelial cell lines HCT116, HT-29, and LoVo contained high levels of CBS when compared with a non-malignant colonic mucosal cell line (NCM356). In contrast to CBS, no cancer-specific differences could be detected in the expression of CSE, or the third H2S-producing enzyme, 3-mercaptopyruvate sulfurtransferase (3-MST). The marked and selective upregulation of CBS in tumor cells induced us to re-focus on the functional role of this enzyme in cancer. shRNA-mediated silencing of CBS significantly reduced HCT116 cell H2S production, proliferation, migration, and invasion and attenuated endothelial cell migration in tumor/endothelial cell co-cultures in vitro.Citation4 Subsequent studies in nude mice bearing subcutaneous tumor xenografts of HCT116 cells with stable lentiviral silencing of CBS exhibited a reduced rate of tumor growth compared with control HCT116 cells.Citation4 Furthermore, CBS silencing in cancer cells reduced intratumoral neovessel density, suggesting that H2S diffuses out from the tumor cell to stimulated tumor angiogenesis. The effects of CBS silencing were recapitulated with the pharmacological CBS inhibitor aminooxyacetic acid (AOAA) both in vitro and in vivo.Citation4 Moreover, treatment of nude mice with AOAA attenuated the growth of patient-derived colon cancer xenografts and reduced peritumoral blood flow.Citation4
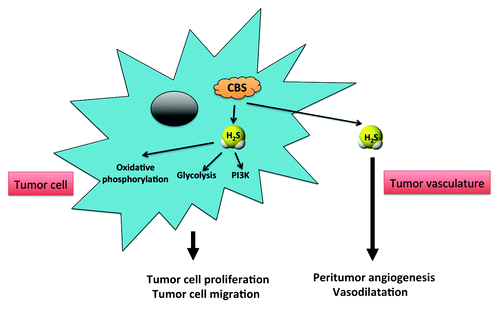
Based on the above data we have formulated a new concept, which implicates tumor-produced CBS-derived H2S as a combined autocrine and paracrine-signaling molecule (). As an autocrine factor, H2S stimulates growth, proliferation, and migration of the colorectal cancer cell, while as a paracrine factor it promotes tumor angiogenesis and peritumoral vasodilation.
Figure 1. Stimulation of colorectal cancer cell growth, proliferation, migration, and peritumor angiogenesis and blood flow by the CBS/H2S axis. CBS utilizes l-cysteine and L-homocysteine to produce H2S in colorectal cancer cells. H2S acts as a mitochondrial electron donor, thereby stimulating oxidative phosphorylation. In addition H2S stimulates glycolysis via activation of GAPDH, most likely via its post-translational modification (sulfhydration). These responses facilitate ATP production, which is essential for growth, proliferation, and cell movement. Moreover, CBS-derived H2S may act an endogenous activator of the PI3K pathway, a classical pro-growth signaling pathway. Tumor-derived H2S may also diffuse out of the tumor tissue, thereby reaching peritumoral and intratumoral vascular tissue, where it increases the supply of the tumor cell with blood and nutritients via stimulation of neovessel formation (tumor angiogenesis) as well as by acting as a local vasodilator hormone.
In contrast to CBS, our data showed that silencing of CSE or its inhibition with propargylglycine (PAG) does not inhibit the growth of HCT116 cells.Citation4 Thus, CBS, and not CSE is the relevant source of endogenous, tumor cell-produced H2S. While the potential role of endothelial-produced, CSE-derived H2S in tumor angiogenesis requires further investigation, our in vivo data in the nude mice showing that the CSE inhibitor PAG does not inhibit tumor growthCitation4 suggest that CSE-derived H2S does not play a major role (at least in colorectal cancers). We hypothesize that this may be due to the fact that, due to the massive upregulation of CBS in the tumor tissue—the peritumor vasculature is already in a “cloud” of H2S; if the endothelial cells are already exposed to high amounts of H2S produced by the tumor tissue, than supplemental, intraendothelial production of H2S may only have a minor effect on endothelial cell H2S homeostasis.
What are the molecular mechanisms by which the CBS/H2S axis stimulates the growth, proliferation, and migration of colorectal cancer cells? With regard to cancer cell proliferation, one mechanism is related to the role of H2S as a bioenergetic factor. Recent data demonstrate that H2S, at relatively low concentrations, acts as a mitochondrial electron donor, which works in concert with the “classical” Krebs-cycle-derived electron donors.Citation5,Citation6 A separate line of studies identified H2S as an activator the glycolytic enzyme GAPH via sulfhydration.Citation7 In line with these findings, we showed that silencing of CBS and inhibition of cell proliferation is associated with an attenuation of both oxidative phosphorylation and glycolysis in HCT116 cells.Citation4 Another mechanism may be related to a H2S-mediated activation of PI3K: An independent line of studies in HCT116 cells demonstrates that H2S is a potent activator of the PI3K pathway;Citation8 it also regulates cell cycle via the phosphorylation of cell cycle kinase inhibitor p21CIP.Citation8 While the precise molecular relationship between these pathways remains to be delineated, it is conceivable that the energetic and signaling responses act in functional synergy for tumor cell movement by providing “direction” (the PI3K pathway contributes to cell polarity and guides the direction of migration) and “fuel” (ATP, to help in the “execution” of the process), respectively.
Taken together, CBS-derived H2S emerges as a novel endogenous tumor cell-derived growth and survival factor. Further work will determine whether small-molecule CBS inhibitors (on their own, or perhaps in an appropriately chosen combination with other anticancer drugs) can be of utility as anticancer agents. In order to identify the most effective combinations, the relationship of H2S-mediated pathways with “traditional” bioenergetic and signaling pathways needs to be characterized in detail. When considering the translational aspects of our discovery, the physiological roles of CBS (including homocysteine metabolism and the regulation of neurotransmission), and the potential adverse consequences of its pharmacological inhibition need to be taken into account as well.
Disclosure of Potential Conflicts of Interest
Drs Hellmich and Szabo are founders, directors, and stockholders of CBS Therapeutics, a for-profit company involved in the research and development of CBS inhibitors for cancer therapy.
References
- Szabo C. Sci Transl Med 2010; 2:59ps54; http://dx.doi.org/10.1126/scitranslmed.3000721; PMID: 21106939
- Wang R. Physiol Rev 2012; 92:791 - 896; http://dx.doi.org/10.1152/physrev.00017.2011; PMID: 22535897
- Papapetropoulos A, et al. Proc Natl Acad Sci U S A 2009; 106:21972 - 7; http://dx.doi.org/10.1073/pnas.0908047106; PMID: 19955410
- Szabo C, et al. Proc Natl Acad Sci U S A 2013; 110:12474 - 9; http://dx.doi.org/10.1073/pnas.1306241110; PMID: 23836652
- Goubern M, et al. FASEB J 2007; 21:1699 - 706; http://dx.doi.org/10.1096/fj.06-7407com; PMID: 17314140
- Módis K, et al. FASEB J 2013; 27:601 - 11; http://dx.doi.org/10.1096/fj.12-216507; PMID: 23104984
- Mustafa AK, et al. Sci Signal 2009; 2:ra72; http://dx.doi.org/10.1126/scisignal.2000464; PMID: 19903941
- Cai WJ, et al. Cell Biol Int 2010; 34:565 - 72; http://dx.doi.org/10.1042/CBI20090368; PMID: 20184555