In the cerebral cortex, GABAergic interneurons play critical roles in regulating the output of individual excitatory pyramidal neurons as well as synchronizing the outputs of pyramidal neuron ensembles. In both mice and humans, many cortical interneurons originate in a subcortical embryonic structure, the medial ganglionic eminence (MGE), and fall into 1 of 2 non-overlapping categories defined by their expression of either parvalbumin (PV) or somatostatin (SST). Deficits in either of these subpopulations have been implicated in a variety of neuropsychiatric disorders, including schizophrenia, autism and epilepsy. Thus, the derivation of these neurons from human stem cells is an invaluable advance to the study of the molecular mechanisms underlying the pathogenesis of cortical interneuron-related disorders in humans. Furthermore, cortical interneurons have the remarkable ability to survive, migrate and integrate into neonatal or adult CNS post-transplantation, making them attractive candidates for usage in cell-based therapies for neuropsychiatric disorders.
Recently there has been a flourish of progress in generating cortical interneurons from both mouse and human embryonic stem cells (ESC)s. Much of this progress can be attributed to the use of BAC transgenic or homologous recombination approaches to insert the fluorescent reporter GFP under control of Nkx2.1 or Lhx6, transcription factors expressed in MGE progenitors or post-mitotic interneurons, respectively. Following the pioneering work of Sasai,Citation1 Maroof et al. used an Lhx6-GFP mouse ESC line to demonstrate that mouse embryonic stem cells (mESCs) can be differentiated into both PV+ and SST+ cortical interneurons.Citation2 Importantly, 30 d after transplantation into neonatal neocortex, these cells were found to display the typical fast-spiking or burst-spiking responses to depolarizing current expected for PV and SST-expressing interneurons, respectively.Citation2 Similar results inducing MGE fates were achieved using a FoxG1::Venus ESC line.Citation3 However, at this point no study of stem-cell derived interneurons has demonstrated their capacity to show native-like axonal targeting properties, such as a tendency to target the cell body and proximal dendrites of pyramidal neurons for PV+ interneurons, or the tendency to target more distal dendrites for SST+ interneurons.
While studies differentiating human stem cells into putative cortical interneurons have yet to demonstrate either subgroup-selective axon targeting or spiking properties, major progress has recently been made using a human ES line in which GFP has been knocked into the Nkx2.1 locus.Citation4 Since the initial study of this line used a relatively low-efficiency, retinoic-acid based protocol to generate SST+, interneuron-like cells identified after culture with mouse cortical cells, 3 recent papers have used this line in demonstrations of cortical interneuron differentiation.Citation5-Citation7 While a detailed comparison of these studies is well beyond the scope of this piece, a summary of their collective progress and shortcomings is warranted. Key aspects of progress include (1) the efficient generation of Nkx2.1-Foxg1 pallidal telencephalon;Citation5-Citation7 (2) the generation of cells that express cortical interneuron markers within extensions of the initial culture;Citation7 (3) migration from MGE to cortex on mouse slices;Citation5 (4) PV and SST differentiation,Citation5,Citation6 as well as inputCitation5 and outputCitation5,Citation6 synaptogenesis in co-cultures with dissociated cells from mouse cortexCitation5 or on cortical astrocytes.Citation6 Of note, interneuron maturation appeared to occur far more rapidly for human MGE-like progenitors when cultured on the dissociated cortex of mouse embryos (4 weeks)Citation5 vs. cortical astrocytes (20–30 weeks).Citation6 That said, a direct and carefully controlled comparison would be needed to confirm this seemingly important difference; (5) tangential migration following transplant into neonatal neocortex,Citation5,Citation6 with survival up to 7 mo,Citation6 and evidence that the transplanted neurons receive excitatory inputs.Citation6 Finally, an additional study used a protocol similar to those implemented above to generate Nkx2.1+ cells that, following transplantation into adult mouse hippocampus, altered hippocampal electrophysiology and function.Citation8 However, while promising, in this study transplantation included cholinergic and GABAergic neurons, and interneuron-selective differentiation of the GABAergic neurons was not demonstrated.
Collectively, these studies lay a solid foundation for using human stem cells in the study of ventral forebrain progenitor fate determination, as well as the initial fate determination of GABAergic interneurons, their intrinsic mechanisms of tangential migration, migration guidance, and their input/ouptut synaptogenesis. Each of the above areas has associations to neurodevelopmental disorders involving specific genes and proteins. Despite these advances, major shortcomings to realizing the full potential of human stem cell-derived cortical interneurons remain. Key among these is the protracted maturation of stem cell derived interneurons following xenographic transplantation into mouse neocortex (). While 6 mo may be an expensive but workable maturation time for basic studies of human interneuron maturation, for initial trials of cell-based therapy for intractable seizures, this delay, given the likelihood that such trials would involve patients at acute risk of death, may render such trials untenable. Unfortunately, human interneurons follow a similarly protracted course of maturation during their native development. Thus, studies of the regulation of interneuron maturation, and the identification of methods to induce precocious maturation, may be required to bring cell based therapy using human stem cell derived interneurons closer to a clinical reality.
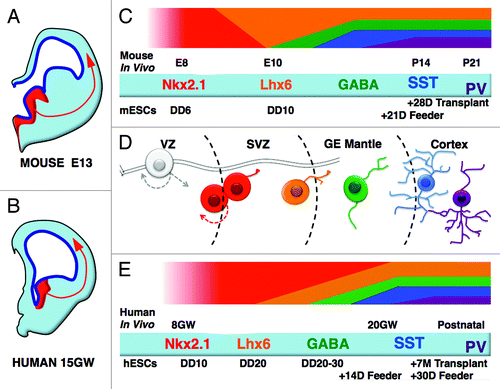
Figure 1. Comparison of mouse and human in vivo and ESC-based in vitro PV and SST interneuron development. Schematic of a coronal hemisection through the embryonic mouse (A) and human (B) forebrain at comparable ages of development, embryonic day 13.5 and 15 gestational weeks (not to scale). Shown in red is the Nkx2.1-expressing medial ganglionic eminence (MGE). The MGE is the progenitor domain for most PV- or SST-expressing cortical interneurons and is well conserved in mammals. Mouse ESC-derived PV- and SST-expressing cells mature at analogous rates, with both makers detectable by approximately 4 wk, either on mouse cortex co-cultures or after transplantation into mouse neonatal cortex (C and D). Conversely human ESC-derived PV- and SST-expressing cells mature very slowly after transplantation, similarly to their in vivo counterparts; however, rapid maturation is facilitated via co-culture with mouse neocortical cells in vitro (D and E). E, embryonic day; DD, differentiation day; GW, gestational weeks; P, days after birth; D, days beyond DD on feeder.
References
- Watanabe K, et al. Nat Neurosci 2005; 8:288 - 96; http://dx.doi.org/10.1038/nn1402; PMID: 15696161
- Maroof AM, et al. J Neurosci 2010; 30:4667 - 75; http://dx.doi.org/10.1523/JNEUROSCI.4255-09.2010; PMID: 20357117
- Danjo T, et al. J Neurosci 2011; 31:1919 - 33; http://dx.doi.org/10.1523/JNEUROSCI.5128-10.2011; PMID: 21289201
- Goulburn AL, et al. Stem Cells 2010; 29:462 - 73; http://dx.doi.org/10.1002/stem.587
- Maroof AM, et al. Cell Stem Cell 2013; 12:559 - 72; http://dx.doi.org/10.1016/j.stem.2013.04.008; PMID: 23642365
- Nicholas CR, et al. Cell Stem Cell 2013; 12:573 - 86; http://dx.doi.org/10.1016/j.stem.2013.04.005; PMID: 23642366
- Germain ND, et al. Stem Cells Dev 2013; 22:1477 - 89; http://dx.doi.org/10.1089/scd.2012.0264; PMID: 23351095
- Liu Y, et al. Nat Biotechnol 2013; 31:440 - 7; http://dx.doi.org/10.1038/nbt.2565; PMID: 23604284