Abstract
5-methylcytosine is an important epigenetic modification involved in gene control in vertebrates and many other complex living organisms. Its presence in Drosophila has been a matter of debate and recent bisulfite sequencing studies of early-stage fly embryos have concluded that the genome of Drosophila is essentially unmethylated. However, as we outline here, the Drosophila genome harbors a well-conserved homolog of the TET protein family. The mammalian orthologs TET1/2/3 are known to convert 5-methylcytosine into 5-hydroxymethylcytosine. We discuss several possible explanations for these seemingly contradictory findings. One possibility is that the 2 modified cytosine bases are generated in Drosophila only at certain developmental stages and in a cell type-specific manner during neurogenesis. Alternatively, Drosophila Tet and its mammalian homologs may carry out catalytic activity-independent functions, and the possibility that these proteins may oxidize 5-methylcytosine in RNA created by the methyltransferase Dnmt2 should also be strongly considered.
Introduction
Modified DNA bases exist in the genomes of a wide array of organisms, including bacteria, fungi, plants, and animals. These modified bases usually escape detection by standard DNA sequencing approaches, and therefore their nature, prevalence, tissue specificity, and DNA sequence-dependent distribution has remained largely unexplored. All studied vertebrate genomes contain the base 5-methylcytosine (5mC) at levels equivalent to a few % of all cytosines present in that organism.Citation1 However, 5mC is present at variable and often very low to non-detectable levels in invertebrates. This modification arises in a post-replicative enzymatic reaction, when DNA methyltransferase (Dnmt) proteins transfer a methyl group from the cofactor S-adenosyl-L-methionine onto carbon 5 of cytosine in DNA. The primary target sequence in mammalian cells is the CpG dinucleotide. Paradoxically, even though methylation of cytosine is highly conserved in vertebrates, its presence at CpG dinucleotides has a substantial mutagenic effect, which has led to a strong depletion of CpG sequences during evolution.Citation2 When CpG sequences are unmethylated, however, they are not mutated, are maintained in the germ line, and have accumulated in particular compartments of the genome termed CpG islands. The evolutionary conservation of DNA methylation despite its inherent mutagenicity attests to its likely biological importance, which after decades of research, is still not understood in complete detail.
DNA Methylation in Mammals
In mammalian cells, methylation of cytosines is chiefly performed by 3 catalytically active DNA methyltransferases, the maintenance methyltransferase DNMT1, which acts predominantly on hemimethylated CpG sites arising during DNA replication, and the DNA methyltransferases DNMT3A and DNMT3B, which operate in de novo methylation of previously unmethylated CpG sequences but also in the maintenance of DNA methylation patterns.Citation3,Citation4 A catalytically inactive DNMT3-like protein, DNMT3L, forms heterodimers with and stimulates the de novo methylation activity of DNMT3A and DNMT3B.Citation5-Citation7 There is a fourth DNA methyltransferase gene in mammalian genomes, termed DNMT2, which was initially identified using homology searches with known DNA methyltransferase sequences.Citation8,Citation9 It is well understood that deletion of the Dnmt genes Dnmt1, Dnmt3a, or Dnmt3b in the mouse results in lethality and severe developmental defects,Citation10,Citation11 whereas deletion of Dnmt2 in mice has no recognizable phenotype.Citation12 The major functional role of DNA methylation in vertebrate development is thought to be the suppression of inappropriate transcription, which is accomplished by different mechanisms, such as interference with transcription factor binding or modulation of chromatin structure mediated by proteins that interact with CpG-methylated DNA.Citation13
DNA Methylation in Insects
For well over 2 decades, there has been much debate as to whether there is cytosine methylation present in the Drosophila melanogaster genome. Initial studies performed in the 1980s produced conflicting results.Citation14,Citation15 This organism lacks homologs of the mammalian DNA methyltransferase genes Dnmt1, Dnmt3a, or Dnmt3b but contains a gene encoding a homolog of Dnmt2 (also known as Mt2; NM_058127). The Drosophila genome also harbors a homolog of the mammalian methylated-CpG binding protein MBD2 (dMBD2/3). The dMBD2/3 protein was shown to have the capacity to bind to CpG-methylated DNA, at least in vitro.Citation16,Citation17 In the year 2000, Lyko et al. reported that Drosophila DNA is methylated, but that Drosophila genome methylation is restricted to early stages of embryo development.Citation18 Confocal analysis of immunostained Drosophila embryos provided further evidence for the methylation of embryonic DNA.Citation19 Following this initial work, a more region-specific distribution of 5mC in the Drosophila genome has been suggested by studies using single-nucleotide resolution bisulfite sequencing for DNA methylation analysis.Citation20 However, these results have been controversially debated in the literature.Citation21,Citation22
In a very recent publication, the conclusion was reached that the genomes of organisms that contain a Dnmt2 gene but do not harbor Dnmt1 or Dnmt3a/b, such as Drosophila, lack DNA methylation altogether.Citation23 This difference in results was attributed to a lack of specificity of the earlier applied methods in comparison to the highly selective and comprehensive whole genome bisulfite sequencing used in the latest publication. However there are numerous reported cases of insects, including ants, aphids, bees, flies, and beetles (Acyrthosiphon pisum, Aedes aegypti, Apis mellifera, Camponotus floridanus, Harpegnathos saltator, Nasonia vitripennis, Tribolium castaneum) having clearly detectable levels and specific patterns of 5mC,Citation24-Citation31 which would mean that the absence of DNA methylation in Drosophila melanogaster would seemingly be unique within this class of animals. Particularly well-studied cases can be found with honeybees and antsCitation25,Citation32 whose epigenetic machinery has much more in common with mammals than that found in Drosophila. These insects contain Dnmt1- and Dnmt3-like enzymes. The Apis mellifera DNA methylation has been shown be to responsible for the generation of the different colony phenotypes in bees.Citation28
CpG Dinucleotide Frequencies within Drosophila Genes
We first asked if it is possible to obtain clues about the presence or absence of DNA methylation in Drosophila by considering sequence features of the Drosophila genome. As mentioned earlier, CpG dinucleotides have eroded over an evolutionary time scale, leading to a current level of observed vs. expected CpG frequencies of only 0.2 to 0.25 in mammals, in other words, around 80% of these sites have been lost during mammalian evolution. CpG depletion is not observed in Drosophila genes, in which the observed-to-expected CpG frequency is very close to 1. Assuming that CpG depletion is caused by mutational mechanisms, including deamination of 5-methylcytosine,Citation2 one would therefore predict that the genome of Drosophila contains little to no 5mC. However, this prediction would only apply to the germ line and not to somatic cells or tissues, which might still harbor methylated sequences. CpG islands, the typical GC-rich and not CpG-depleted areas of vertebrate genomes, which are often important for gene control, are not present in the Drosophila genome, perhaps owing to its already higher general CpG frequency.Citation33 In contrast to flies, the observed to expected ratio of CpGs in the honey bee (Apis mellifera) shows a striking bimodal distribution clearly suggesting that the genome of bees contains methylated and unmethylated genes.Citation25 In mammals, different promoter classes have been identified. Among these, the so called “broad promoters” have multiple transcription start sites, are rich in CpG sequences, are generally embedded within large CpG islands, and are controlled often by epigenetic modifications, including DNA methylation and histone modifications. Other types of promoters including those having sharp transcription start sites usually are not within CpG islands and are less sensitive to such epigenome modifications. Surprisingly, Drosophila has both the “broad” and “sharp” promoters, suggesting that this bimodal architecture of promoters is conserved.Citation33,Citation34 This would suggest that either (1) there is such epigenomic modulation also in Drosophila, but we have not detected it, or (2) there are other mechanisms that replace CpG methylation in the regulation of those broad promoters.
The Enigmatic Dnmt2
There are some well-studied organisms, including Saccharomyces cerevisiae and Caenorhabditis elegans, which do not contain any DNA methyltransferase gene, and there is agreement that these 2 organisms also do not contain DNA cytosine methylation. Several organisms, including Drosophila melanogaster and Schistosoma mansoni, contain only a Dnmt2 homolog. It is now recognized that Dnmt2 is primarily an RNA methyltransferase since it has been convincingly shown that Dnmt2 methylates the cytosine at position 38 in tRNAAsp.Citation12 However, earlier studies with DNA substrates in vitroCitation35 have revealed some limited activity of Dnmt2 on DNA. According to biochemical fractionation studies, the Dnmt2 protein is present in both cytoplasm and nucleus, where it resides in the nuclear matrix, and therefore it could theoretically act on RNA and/or DNA.Citation36 Dnmt2 shows little sequence homology with known RNA methyltransferasesCitation37,Citation38 and uses a DNA methyltransferase-type mechanism to methylate tRNA.Citation39 Furthermore, the catalytic active site amino acids typical of DNA methyltransferases, including the critical adjacent proline–cysteine residues, are all present in Dnmt2.Citation3 Collectively, the currently available experimental evidence favors a role of Dnmt2 in RNA methylation, although activity on DNA cannot categorically be excluded.
TET Proteins and 5-Hydroxymethylcytosine
The ten-11 translocation (TET) family of genes belong to a larger family of Fe2+- and 2-oxoglutarate-dependent dioxygenases, which were found to be capable of oxidizing 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) and subsequently of further oxidation of 5hmC to 5-formylcytosine (5foC) and terminally to 5-carboxlycytosine (5caC).Citation40-Citation43 Since the identification of these additional layers of complexity upon the single original DNA modification (5mC), there has been much interest in identifying possible roles that these new modifications might play during cellular development and differentiation.Citation44-Citation46 Much of the currently available evidence suggests that 5hmC is important in the regulation of transcription and in developmental remodeling of DNA methylation patterns when 5mC oxidation appears to be an initial step toward genome-wide DNA demethylation.Citation47-Citation49
TET Family Proteins and Orthology in Drosophila and Other Metazoans
It has been previously reported that at least one member of the TET family is present in metazoans.Citation50 Mammalian genomes encode 3 different 5mC oxidases, TET1, TET2, and TET3. Unexpectedly, the genome of Drosophila melanogaster also contains a TET homolog. The TET proteins can be characterized by the presence of 2 conserved core domains, an N-terminal CXXC zinc-finger DNA binding domain and a C-terminal Fe2+- and 2-oxoglutarate-dependent dioxygenase (Tet_JBP) catalytic domain (). This excludes TET2 orthologs, which are believed to have lost the CXXC domain via a chromosomal inversion.Citation51 TET3 encodes several catalytically active isoforms that differ in the presence or absence of the N-terminal CXXC domain (S-G Jin and GP Pfeifer, unpublished results). Interestingly, a TET protein homolog is present in the basal placozoan Tricoplax adhaerens (XM_002108929) and the even more basal sponge Amphimedon queenslandica (XP_003384410). Indeed, surprisingly, the sponge protein is more similar to the human, than the Trichoplax is to the human TET proteins. The CXXC DNA binding domain has long been known to be responsible for CXXC domain containing proteins’ ability to bind to unmethylated cytosines in the context of CpG dinucleotides.Citation52 These domains are characterized by 8 conserved cysteine residues. Crystallography studies have revealed a pair of histidine and glutamine residues, which are responsible for the majority of interactions with CpG dinucleotides during DNA binding, and which are also evolutionally conserved.Citation53-Citation55 The catalytic domain of TET proteins can itself be divided into an N-terminal and C-terminal portion, which contain two halves of the conserved catalytic (HxD/H) triad separated by a lengthy, low-complexity, non-conserved linker region.Citation50 The conserved core domains distinguish the TET family of dioxygenases from other members of the dioxygenase superfamily by the presence of the CXXC DNA binding domain, and it is the combination of all of the domains that characterizes a “TET” protein. Organisms can generally be split into evolutionary groups based on the number of TET genes identified in their genomes. The first group, mammals and vertebrates, have 3 orthologs of TET genes, except X. tropicalis (a model organism), which only has 2 identifiable members orthologous to TET2 and TET3. The second group is all other metazoans from Trichoplax onwards, such as arthropods, including the aforementioned honeybees and ants, which all contain a single TET gene, and, despite evolutionary divergence, their TET proteins are very highly orthologous to the mammalian TET proteins in their previously mentioned core domains. One notable exception of the presence of a TET protein can be found in the model organism Caenorhabditis elegans, which, like other nematodes, contains no identifiable TET genes, but also has no DNMT genes and no detectable genomic DNA methylation.
Figure 1. Domain architecture of human TET1, TET2, and TET3 and Drosophila melanogaster dTet. The conserved CXXC, cysteine-rich region and catalytic domain regions are indicated in blue, orange, and green, respectively.
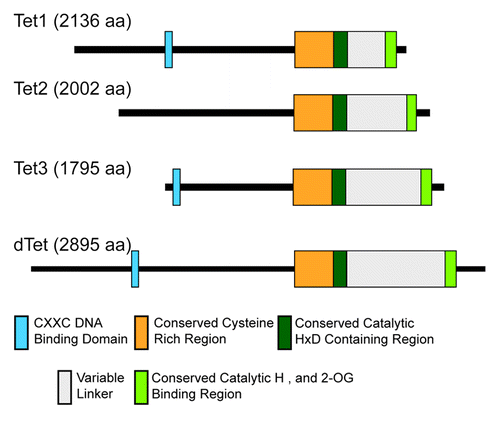
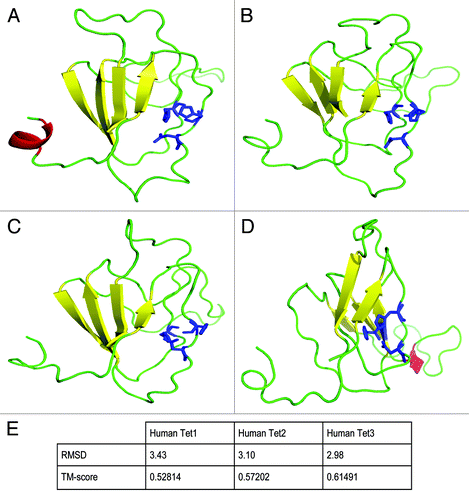
The alignments () show that the evolutionary conservation of these non-variable domains, the CXXC domain and the dioxygenase domain, is remarkably high between humans, mouse, Xenopus tropicalis, Apis mellifera, and Drosophila melanogaster. An additional ~200 amino acid region just upstream of the N-terminal half of the dioxygenase domain is also highly conserved between the Drosophila and the mammalian proteins. Three-dimensional modeling and comparisons between human TET1, TET2, and TET3 and Drosophila Tet (dTet) also indicate a high degree of similarity between all 4 proteins for the catalytic domain including the conserved metal binding domain residues (). According to RSMD and TM scores, Drosophila Tet is most closely related to human TET3 ().
Figure 2. Alignment of the conserved residues from the functional domains of TET proteins from diverse multicellular organisms. (A) Alignment of the CXXC DNA binding domains from human, Xenopus tropicalis, Mus musculus, Drosophila melanogaster, and Apis mellifera TET genes. Conserved domain residues are highlighted in blue. The residues predicted to play a role in DNA binding from previous crystal structure data of MLL1 and Cfp1 are indicated in brown. (B and C) Alignment of the conserved HxD and H residues of the Fe2+ and 2-oxoglutarate binding sites comprising the active site from human, Xenopus, mouse, Drosophila, and Apis TET genes. The conserved catalytic residues are indicated in green and orange.
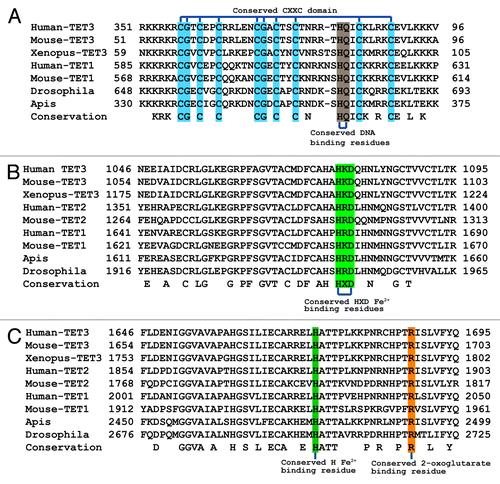
Figure 3. 3D modeling of the metal binding domain of human TET proteins and Drosophila Tet. The 3D models of human TET1 (A), human TET2 (B), human TET3 (C), and dTet (D) conserved sub-sequences including the metal binding residues of the catalytic domain are shown. Models were based on the template 3s57 chain A of the human ABH2 protein and were built and evaluated using the IntFOLD, I-TASSER and ModFOLD4 servers and the SPARKS-X and Modeler standalone programs.Citation73-Citation76 Details of the modeling approach are provided in the Supplementary Material. The images of models were rendered using PyMOL. The conserved metal binding residues are indicated as blue sticks; β-sheets are in yellow; helices in red; and loop regions are shown in green. (E) Root-mean-square deviation (RMSD) scores (lower is closer) and template modeling TM scores (higher is closer) indicating the structural relationship between human TET 3D models and the Drosophila Tet 3D model. The TM-align methodCitation77 was used to score the structural relationships between models. The TM scores in indicate that all of the human Tet models are likely to share the same fold as the Drosophila Tet model, as they are above the 0.5 threshold therefore indicating a significant match.Citation78 Both the RMSD and the TM scores indicate that the Human Tet3 model is perhaps the closest to the Drosophila Tet model in terms of global structural similarity.
The presence of these proteins in almost every multicellular organism coupled with their very highly conserved domains clearly implies that these proteins’ enzymatic activity must be equally highly conserved having evolved at an early stage in metazoan evolution, prior to tissue differentiation. In mammals, mainly humans and mice, the function of 5hmC, the major product of TET protein activity, is becoming clearer with the rapid pace of new studies with suggested roles in gene regulation, mammalian development, differentiation, and disease states.Citation44 In other organisms the function of TET proteins has rarely been studied and, in many cases, only mentioned in passing. Drosophila melanogaster, as a “model organism" is well known as having a close ortholog of the mammalian DNMT2 enzyme, but as mentioned earlier, no homologs of either DNMT1 or DNMT3A and DNMT3B are present. However, DNMT2 is now recognized as a tRNA methyltransferase and is believed not to be acting on DNA.Citation12 The presence of a Tet gene in Drosophila melanogaster is therefore puzzling and leads to the interesting question of what is the function of a 5-methylcytosine oxidase in this organism if there is no genomic 5mC?
Why Is There a 5mC Oxidase, If There Is No 5mC?
Given the fact that there is a clearly identifiable Drosophila Tet protein (; NM_001259652) the argument for the presence of methylation in this species takes on another dimension with the realization that, as dTet will very likely act on 5mC and oxidize it, there is no reason for dTet to be conserved in the Drosophila genome, if there is no 5mC for it to act upon. Since the substrate for the TET proteins is genomic 5mC, it seems highly unlikely that Drosophila would have maintained a protein the size of dTet if, as is becoming more apparent in mammalian studies, it was not playing a significant role at one or more points in development as an epigenetic modifier. From the alignments () the residues for DNA binding and catalytic activity are all very highly conserved, indicating that it is also less likely that dTet would have evolved a different function than that which has been previously reported for mammalian TETs. Given the obvious conservation of important residues, domains, and protein structure, the complete lack of 5mC in Drosophila, as now suggested by Raddatz et al.,Citation23 seems very unexpected.
Expression and Tissue Distribution of dTet and Dnmt2
The bisulfite-sequencing method used by Raddatz et al.Citation23 would have detected both 5hmC and 5mC due to its inability to distinguish between the 2 base modifications.Citation56,Citation57 It may be possible that some highly repetitive regions of the Drosophila genome could contain 5mC/5hmC but would have been excluded from the whole genome analysis. In those cases where 5mC has been reported in Drosophila, it has been at very low levels. It thus appears that levels of 5mC, if present at all, are either very low, limited to specific genomic locations, limited to specific cell types or developmental phases, or possibly a combination of all of these factors.
It is clear that there is a conserved Tet gene in Drosophila, and it is also clear that this gene is actively expressed in certain tissues. Examining available expression data for dTet mRNA obtained by in situ hybridization reveals evidence that expression of dTet does not appear until after Drosophila developmental stage 6 (3 h post-fertilization). But, intriguingly, by stage 16 (15 h post fertilization) dTet expression is strongly present in, and limited to, the central nervous system and the brain (http://insitu.fruitfly.org/cgi-bin/ex/report.pl?ftype=1&ftext=CG2083) (note: the old locus tag CG2083 is now referred to as CG43444). Expression of dTet (CG2083) is first detected in the ventral neuroectoderm, and transcripts are also found in neuroblasts, ganglion mother cells, and neurons of the central nervous system. By stage 13, expression is observed only in the nervous system.Citation58 RNA-seq data from early embryos reveals a similar pattern of dTet expression with a peak during the development stages between 6–8 h ().Citation59 This tissue specificity mirrors the expression pattern in mice, where Tet3 expression has been reported to be high in the developing brain,Citation60 the tissue which also has the highest reported levels of 5hmC.Citation61 Tissue expression data from later stages of development, including larvae and adults, show that there are still high levels of dTet present in the brain and central nervous system when compared with other tissues such as salivary gland and the digestive system (). From the expression data, it can be predicted that dTet could be playing an important role necessary for brain and central nervous system development. In developing mouse embryos, Tet proteins and 5hmC are functionally involved in neuronal differentiation.Citation60 Interestingly, in Drosophila embryos, expression of Dnmt2 follows a similar expression profile, albeit levels of Dnmt2 RNA are scored as lower than those of dTet (). From these data, it appears that Dnmt2 and dTet expression levels are close to zero at embryonic stages 0–2 h, the time point from which Drosophila embryos were collected by Raddatz et al. for bisulfite sequencing analysis.Citation23
Figure 4. Expression of Dnmt2 and dTet during embryonic stages of Drosophila development. The expression patterns of dTet and Dnmt2/Mt2 show a significant increase of dTet expression from the fourth hour of embryo development onwards; this pattern is mirrored by the expression of Dnmt2/Mt2. RNA-seq data were derived from Graveley et al.Citation59 Expression levels are given as average number of fragments (RNA-seq reads) per kilobase of transcript per million fragments mapped.
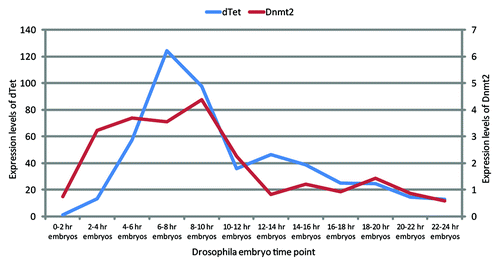
Figure 5. Expression of Dnmt2 and dTet from various Drosophila tissues. These data show that the expression of dTet is highest in the central nervous system. dTet can also be detected in other tissues but at a much lower level. The expression levels of Dnmt2/Mt2 are also highest in the central nervous system though the difference relative to other tissues is smaller.Citation79 Expression levels are given as average number of RNA-seq reads per kilobase of transcript per million fragments mapped.
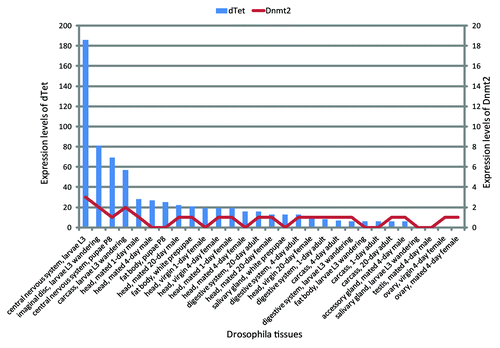
Combination of these data clearly suggests that searches for modified cytosines in Drosophila should include these later developmental time points when the cytosine modifying enzymes are expressed, especially focusing on the central nervous system tissues that show the highest levels of expression of dTet and Dnmt2.
Another recent report could help shed light on where else DNA methylation could be found as well as a potential role for hydroxymethylation. Yadlapalli and Yamashita provided evidence for the non-random segregation of Y and X chromosomes in male Drosophila germline stem cells.Citation62 They show that the non-random chromosome segregation is heritable and is dependent on a wild-type Dnmt2 protein. This would imply that Dnmt2 could be capable of leaving an epigenetic mark on certain chromosomes. Taking a cue from mammalian zygote differential methylation and hydroxymethylation immediately following fertilization, in which paternal DNA from the sperm is converted from 5mC to 5hmC and maternal DNA from the egg is left untouched,Citation48,Citation49 it would seem logical to propose that during this non-random chromosome segregation in Drosophila, there could also be 5mC and/or 5hmC present on one set of chromosomes, which could similarly play a role in segregation.
Alternative Roles of Drosophila Tet?
As summarized earlier, dTet contains several highly conserved TET protein domains, including the CXXC domain, the catalytic dioxygenase domain, and a 200 amino acid region just N-terminal to the first half of the catalytic domain, suggesting that dTet is similar to mammalian TET proteins and may function as a 5mC oxidase. However, current data do not exclude the possibility that dTet would act on 5mC in tRNA, produced by Dnmt2, and would thus be an RNA 5mC oxidase. So far, there have been no reports confirming the existence of 5hmC in RNA, or specifically in tRNA, in any organism. However, there is widespread presence of 5mC not only in tRNA, but also in mRNA and in other non-coding RNA species.Citation63 Since 5hmC in mammalian DNA has remained unconfirmed until 2009,Citation42,Citation64 it would not be too surprising if this base were also present in RNA, and its detection has so far been hampered by the lack of highly sensitive technology, including specific mass spectrometry-based approaches. The dioxygenase domain present in dTet may be capable of 5mC oxidation in RNA. More puzzling is the simultaneous presence of a CXXC domain, a protein module generally thought to interact with unmethylated CpG dinucleotides in DNA.Citation52 However, this domain may also be capable of binding to CpG sites in RNA or has adopted an alternative function, for example by providing a specific structural feature to the protein. Experimental tests with recombinant or overexpressed dTet will need to be conducted to study its ability to oxidize 5mC in tRNA. While there is evidence that 5mC in tRNA may have a stabilizing function and protects it from stress-induced cleavage,Citation65 the biological effect of converting 5mC in tRNA to 5hmC would remain to be determined. Hypothetically, oxidation of 5mC in this tRNA could lead to tRNA destabilization.
Finally, dTet may have evolved a function that is largely independent of its catalytic activity altogether. Mammalian TET proteins have large uncharacterized regions that could potentially interact with other nuclear proteins and may facilitate 5mC-independent functions of TET proteins. However, these regions are poorly conserved between different TET proteins, including dTet. What is known already is that TET proteins can bind to chromatin modifier complexes, including Sin3A, NuRD, and O-linked β-N-acetylglucosamine (O-GlcNAc) transferase (OGT).Citation66-Citation70 Although the precise meaning of these interactions are currently not well understood, it is possible that analogous or even novel functions have evolved in dTet to maintain an alternative epigenetic role of the protein in the absence of 5mC.
Conclusion
In conclusion, we clearly show that a Tet gene is expressed along with Dnmt2 in the Drosophila genome in specific cells and development states, and by inference, the possibility for the presence of 5mC and 5hmC should be considered in those same cells and tissues. We also suggest that any examination of DNA methylation in Drosophila or other organisms where a TET gene is present needs to take into account the likely presence of 5hmC generated by the conserved TET protein(s) and ensure that appropriate methods for the differential examination of both 5mC and 5hmC be used.Citation71,Citation72 The tissues and stages at which Drosophila Tet is expressed would be ideal targets for use to develop a better understanding of any possible DNA-based epigenetic mechanisms present in this organism. Alternative possibilities, specifically that dTet and its mammalian counterparts have functional properties as tRNA 5mC oxidases producing 5hmC in tRNA, should also strongly be considered.
Additional material
Download Zip (117.8 KB)Acknowledgments
Work of the authors was supported by NIH grant CA160965 to GPP.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cc/article/26540
References
- Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 2008; 9:465 - 76; http://dx.doi.org/10.1038/nrg2341; PMID: 18463664
- Pfeifer GP. Mutagenesis at methylated CpG sequences. Curr Top Microbiol Immunol 2006; 301:259 - 81; http://dx.doi.org/10.1007/3-540-31390-7_10; PMID: 16570852
- Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem 2005; 74:481 - 514; http://dx.doi.org/10.1146/annurev.biochem.74.010904.153721; PMID: 15952895
- Jones PA, Liang G. Rethinking how DNA methylation patterns are maintained. Nat Rev Genet 2009; 10:805 - 11; http://dx.doi.org/10.1038/nrg2651; PMID: 19789556
- Chedin F, Lieber MR, Hsieh CL. The DNA methyltransferase-like protein DNMT3L stimulates de novo methylation by Dnmt3a. Proc Natl Acad Sci U S A 2002; 99:16916 - 21; http://dx.doi.org/10.1073/pnas.262443999; PMID: 12481029
- Gowher H, Liebert K, Hermann A, Xu G, Jeltsch A. Mechanism of stimulation of catalytic activity of Dnmt3A and Dnmt3B DNA-(cytosine-C5)-methyltransferases by Dnmt3L. J Biol Chem 2005; 280:13341 - 8; http://dx.doi.org/10.1074/jbc.M413412200; PMID: 15671018
- Xie ZH, Huang YN, Chen ZX, Riggs AD, Ding JP, Gowher H, Jeltsch A, Sasaki H, Hata K, Xu GL. Mutations in DNA methyltransferase DNMT3B in ICF syndrome affect its regulation by DNMT3L. Hum Mol Genet 2006; 15:1375 - 85; http://dx.doi.org/10.1093/hmg/ddl059; PMID: 16543361
- Okano M, Xie S, Li E. Dnmt2 is not required for de novo and maintenance methylation of viral DNA in embryonic stem cells. Nucleic Acids Res 1998; 26:2536 - 40; http://dx.doi.org/10.1093/nar/26.11.2536; PMID: 9592134
- Yoder JA, Bestor TH. A candidate mammalian DNA methyltransferase related to pmt1p of fission yeast. Hum Mol Genet 1998; 7:279 - 84; http://dx.doi.org/10.1093/hmg/7.2.279; PMID: 9425235
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999; 99:247 - 57; http://dx.doi.org/10.1016/S0092-8674(00)81656-6; PMID: 10555141
- Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 1992; 69:915 - 26; http://dx.doi.org/10.1016/0092-8674(92)90611-F; PMID: 1606615
- Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, Golic KG, Jacobsen SE, Bestor TH. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science 2006; 311:395 - 8; http://dx.doi.org/10.1126/science.1120976; PMID: 16424344
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci 2006; 31:89 - 97; http://dx.doi.org/10.1016/j.tibs.2005.12.008; PMID: 16403636
- Urieli-Shoval S, Gruenbaum Y, Sedat J, Razin A. The absence of detectable methylated bases in Drosophila melanogaster DNA. FEBS Lett 1982; 146:148 - 52; http://dx.doi.org/10.1016/0014-5793(82)80723-0; PMID: 6814955
- Achwal CW, Ganguly P, Chandra HS. Estimation of the amount of 5-methylcytosine in Drosophila melanogaster DNA by amplified ELISA and photoacoustic spectroscopy. EMBO J 1984; 3:263 - 6; PMID: 6232132
- Tweedie S, Ng HH, Barlow AL, Turner BM, Hendrich B, Bird A. Vestiges of a DNA methylation system in Drosophila melanogaster?. Nat Genet 1999; 23:389 - 90; http://dx.doi.org/10.1038/70490; PMID: 10581020
- Roder K, Hung MS, Lee TL, Lin TY, Xiao H, Isobe KI, Juang JL, Shen CJ. Transcriptional repression by Drosophila methyl-CpG-binding proteins. Mol Cell Biol 2000; 20:7401 - 9; http://dx.doi.org/10.1128/MCB.20.19.7401-7409.2000; PMID: 10982856
- Lyko F, Ramsahoye BH, Jaenisch R. DNA methylation in Drosophila melanogaster. Nature 2000; 408:538 - 40; http://dx.doi.org/10.1038/35046205; PMID: 11117732
- Kunert N, Marhold J, Stanke J, Stach D, Lyko FA. A Dnmt2-like protein mediates DNA methylation in Drosophila. Development 2003; 130:5083 - 90; http://dx.doi.org/10.1242/dev.00716; PMID: 12944428
- Phalke S, Nickel O, Walluscheck D, Hortig F, Onorati MC, Reuter G. Retrotransposon silencing and telomere integrity in somatic cells of Drosophila depends on the cytosine-5 methyltransferase DNMT2. Nat Genet 2009; 41:696 - 702; http://dx.doi.org/10.1038/ng.360; PMID: 19412177
- Schaefer M, Lyko F. Lack of evidence for DNA methylation of Invader4 retroelements in Drosophila and implications for Dnmt2-mediated epigenetic regulation. Nat Genet 2010; 42:920 - 1, author reply 921; http://dx.doi.org/10.1038/ng1110-920; PMID: 20980983
- Phalke S, Nickel O, Reuter G. Reply to “Lack of evidence for DNA methylation of Invader4 retroelements in Drosophila and implications for Dnmt2-mediated epigenetic regulation”. Nat Genet 2010; 42:921 - 921; http://dx.doi.org/10.1038/ng1110-921
- Raddatz G, Guzzardo PM, Olova N, Fantappié MR, Rampp M, Schaefer M, Reik W, Hannon GJ, Lyko F. Dnmt2-dependent methylomes lack defined DNA methylation patterns. Proc Natl Acad Sci U S A 2013; 110:8627 - 31; http://dx.doi.org/10.1073/pnas.1306723110; PMID: 23641003
- Bonasio R, Li Q, Lian J, Mutti NS, Jin L, Zhao H, Zhang P, Wen P, Xiang H, Ding Y, et al. Genome-wide and caste-specific DNA methylomes of the ants Camponotus floridanus and Harpegnathos saltator. Curr Biol 2012; 22:1755 - 64; http://dx.doi.org/10.1016/j.cub.2012.07.042; PMID: 22885060
- Elango N, Hunt BG, Goodisman MA, Yi SV. DNA methylation is widespread and associated with differential gene expression in castes of the honeybee, Apis mellifera. Proc Natl Acad Sci U S A 2009; 106:11206 - 11; http://dx.doi.org/10.1073/pnas.0900301106; PMID: 19556545
- Feliciello I, Parazajder J, Akrap I, Ugarković D. First evidence of DNA methylation in insect Tribolium castaneum: Environmental regulation of DNA methylation within heterochromatin. Epigenetics 2013; 8:534 - 41; http://dx.doi.org/10.4161/epi.24507; PMID: 23644818
- Fneich S, Dheilly N, Adema C, Rognon A, Reichelt M, Bulla J, Grunau C, Cosseau C. 5-methyl-cytosine and 5-hydroxy-methyl-cytosine in the genome of Biomphalaria glabrata, a snail intermediate host of Schistosoma mansoni. Parasit Vectors 2013; 6:167; http://dx.doi.org/10.1186/1756-3305-6-167; PMID: 23742053
- Lyko F, Foret S, Kucharski R, Wolf S, Falckenhayn C, Maleszka R. The honey bee epigenomes: differential methylation of brain DNA in queens and workers. PLoS Biol 2010; 8:e1000506; http://dx.doi.org/10.1371/journal.pbio.1000506; PMID: 21072239
- Walsh TK, Brisson JA, Robertson HM, Gordon K, Jaubert-Possamai S, Tagu D, Edwards OR. A functional DNA methylation system in the pea aphid, Acyrthosiphon pisum. Insect Mol Biol 2010; 19:Suppl 2 215 - 28; http://dx.doi.org/10.1111/j.1365-2583.2009.00974.x; PMID: 20482652
- Ye YH, Woolfit M, Huttley GA, Rances E, Caragata EP, Popovici J, et al. Infection with a Virulent Strain of Disrupts Genome Wide-Patterns of Cytosine Methylation in the Mosquito. PLoS ONE 2013; 8:e66482; http://dx.doi.org/10.1371/journal.pone.0066482; PMID: 23840485
- Zwier MV, Verhulst EC, Zwahlen RD, Beukeboom LW, van de Zande L. DNA methylation plays a crucial role during early Nasonia development. Insect Mol Biol 2012; 21:129 - 38; http://dx.doi.org/10.1111/j.1365-2583.2011.01121.x; PMID: 22122805
- Simola DF, Wissler L, Donahue G, Waterhouse RM, Helmkampf M, Roux J, Nygaard S, Glastad KM, Hagen DE, Viljakainen L, et al. Social insect genomes exhibit dramatic evolution in gene composition and regulation while preserving regulatory features linked to sociality. Genome Res 2013; 23:1235 - 47; http://dx.doi.org/10.1101/gr.155408.113; PMID: 23636946
- Lenhard B, Sandelin A, Carninci P. Metazoan promoters: emerging characteristics and insights into transcriptional regulation. Nat Rev Genet 2012; 13:233 - 45; PMID: 22392219
- Hoskins RA, Landolin JM, Brown JB, Sandler JE, Takahashi H, Lassmann T, Yu C, Booth BW, Zhang D, Wan KH, et al. Genome-wide analysis of promoter architecture in Drosophila melanogaster. Genome Res 2011; 21:182 - 92; http://dx.doi.org/10.1101/gr.112466.110; PMID: 21177961
- Jeltsch A, Nellen W, Lyko F. Two substrates are better than one: dual specificities for Dnmt2 methyltransferases. Trends Biochem Sci 2006; 31:306 - 8; http://dx.doi.org/10.1016/j.tibs.2006.04.005; PMID: 16679017
- Schaefer M, Steringer JP, Lyko F. The Drosophila cytosine-5 methyltransferase Dnmt2 is associated with the nuclear matrix and can access DNA during mitosis. PLoS One 2008; 3:e1414; http://dx.doi.org/10.1371/journal.pone.0001414; PMID: 18183295
- Schaefer M, Lyko F. Solving the Dnmt2 enigma. Chromosoma 2010; 119:35 - 40; http://dx.doi.org/10.1007/s00412-009-0240-6; PMID: 19730874
- Jurkowski TP, Jeltsch A. On the evolutionary origin of eukaryotic DNA methyltransferases and Dnmt2. PLoS One 2011; 6:e28104; http://dx.doi.org/10.1371/journal.pone.0028104; PMID: 22140515
- Jurkowski TP, Meusburger M, Phalke S, Helm M, Nellen W, Reuter G, Jeltsch A. Human DNMT2 methylates tRNA(Asp) molecules using a DNA methyltransferase-like catalytic mechanism. RNA 2008; 14:1663 - 70; http://dx.doi.org/10.1261/rna.970408; PMID: 18567810
- Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010; 466:1129 - 33; http://dx.doi.org/10.1038/nature09303; PMID: 20639862
- Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011; 333:1300 - 3; http://dx.doi.org/10.1126/science.1210597; PMID: 21778364
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009; 324:930 - 5; http://dx.doi.org/10.1126/science.1170116; PMID: 19372391
- He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011; 333:1303 - 7; http://dx.doi.org/10.1126/science.1210944; PMID: 21817016
- Pastor WA, Aravind L, Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol 2013; 14:341 - 56; http://dx.doi.org/10.1038/nrm3589; PMID: 23698584
- Pfeifer GP, Kadam S, Jin SG. 5-hydroxymethylcytosine and its potential roles in development and cancer. Epigenetics Chromatin 2013; 6:10; http://dx.doi.org/10.1186/1756-8935-6-10; PMID: 23634848
- Shen L, Zhang Y. 5-Hydroxymethylcytosine: generation, fate, and genomic distribution. Curr Opin Cell Biol 2013; 25:289 - 96; http://dx.doi.org/10.1016/j.ceb.2013.02.017; PMID: 23498661
- Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, Surani MA. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science 2013; 339:448 - 52; http://dx.doi.org/10.1126/science.1229277; PMID: 23223451
- Iqbal K, Jin SG, Pfeifer GP, Szabó PE. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc Natl Acad Sci U S A 2011; 108:3642 - 7; http://dx.doi.org/10.1073/pnas.1014033108; PMID: 21321204
- Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, Arand J, Nakano T, Reik W, Walter J. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat Commun 2011; 2:241; http://dx.doi.org/10.1038/ncomms1240; PMID: 21407207
- Iyer LM, Tahiliani M, Rao A, Aravind L. Prediction of novel families of enzymes involved in oxidative and other complex modifications of bases in nucleic acids. Cell Cycle 2009; 8:1698 - 710; http://dx.doi.org/10.4161/cc.8.11.8580; PMID: 19411852
- Ko M, An J, Bandukwala HS, Chavez L, Aijö T, Pastor WA, Segal MF, Li H, Koh KP, Lähdesmäki H, et al. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature 2013; 497:122 - 6; http://dx.doi.org/10.1038/nature12052; PMID: 23563267
- Long HK, Blackledge NP, Klose RJ. ZF-CxxC domain-containing proteins, CpG islands and the chromatin connection. Biochem Soc Trans 2013; 41:727 - 40; http://dx.doi.org/10.1042/BST20130028; PMID: 23697932
- Xu C, Bian C, Lam R, Dong A, Min J. The structural basis for selective binding of non-methylated CpG islands by the CFP1 CXXC domain. Nat Commun 2011; 2:227; http://dx.doi.org/10.1038/ncomms1237; PMID: 21407193
- Xu Y, Xu C, Kato A, Tempel W, Abreu JG, Bian C, Hu Y, Hu D, Zhao B, Cerovina T, et al. Tet3 CXXC domain and dioxygenase activity cooperatively regulate key genes for Xenopus eye and neural development. Cell 2012; 151:1200 - 13; http://dx.doi.org/10.1016/j.cell.2012.11.014; PMID: 23217707
- Cierpicki T, Risner LE, Grembecka J, Lukasik SM, Popovic R, Omonkowska M, Shultis DD, Zeleznik-Le NJ, Bushweller JH. Structure of the MLL CXXC domain-DNA complex and its functional role in MLL-AF9 leukemia. Nat Struct Mol Biol 2010; 17:62 - 8; http://dx.doi.org/10.1038/nsmb.1714; PMID: 20010842
- Jin SG, Kadam S, Pfeifer GP. Examination of the specificity of DNA methylation profiling techniques towards 5-methylcytosine and 5-hydroxymethylcytosine. Nucleic Acids Res 2010; 38:e125; http://dx.doi.org/10.1093/nar/gkq223; PMID: 20371518
- Huang Y, Pastor WA, Shen Y, Tahiliani M, Liu DR, Rao A. The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing. PLoS One 2010; 5:e8888; http://dx.doi.org/10.1371/journal.pone.0008888; PMID: 20126651
- Brody T, Stivers C, Nagle J, Odenwald WF. Identification of novel Drosophila neural precursor genes using a differential embryonic head cDNA screen. Mech Dev 2002; 113:41 - 59; http://dx.doi.org/10.1016/S0925-4773(02)00010-2; PMID: 11900973
- Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, et al. The developmental transcriptome of Drosophila melanogaster. Nature 2011; 471:473 - 9; http://dx.doi.org/10.1038/nature09715; PMID: 21179090
- Hahn MA, Qiu R, Wu X, Li AX, Zhang H, Wang J, Jui J, Jin SG, Jiang Y, Pfeifer GP, et al. Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. Cell Rep 2013; 3:291 - 300; http://dx.doi.org/10.1016/j.celrep.2013.01.011; PMID: 23403289
- Münzel M, Globisch D, Brückl T, Wagner M, Welzmiller V, Michalakis S, Müller M, Biel M, Carell T. Quantification of the sixth DNA base hydroxymethylcytosine in the brain. Angew Chem Int Ed Engl 2010; 49:5375 - 7; http://dx.doi.org/10.1002/anie.201002033; PMID: 20583021
- Yadlapalli S, Yamashita YM. Chromosome-specific nonrandom sister chromatid segregation during stem-cell division. Nature 2013; 498:251 - 4; http://dx.doi.org/10.1038/nature12106; PMID: 23644460
- Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res 2012; 40:5023 - 33; http://dx.doi.org/10.1093/nar/gks144; PMID: 22344696
- Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 2009; 324:929 - 30; http://dx.doi.org/10.1126/science.1169786; PMID: 19372393
- Schaefer M, Pollex T, Hanna K, Tuorto F, Meusburger M, Helm M, Lyko F. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev 2010; 24:1590 - 5; http://dx.doi.org/10.1101/gad.586710; PMID: 20679393
- Shi FT, Kim H, Lu W, He Q, Liu D, Goodell MA, Wan M, Songyang Z. Ten-eleven translocation 1 (Tet1) is regulated by O-linked N-acetylglucosamine transferase (Ogt) for target gene repression in mouse embryonic stem cells. J Biol Chem 2013; 288:20776 - 84; http://dx.doi.org/10.1074/jbc.M113.460386; PMID: 23729667
- Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PA, Rappsilber J, Helin K. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature 2011; 473:343 - 8; http://dx.doi.org/10.1038/nature10066; PMID: 21490601
- Chen Q, Chen Y, Bian C, Fujiki R, Yu X. TET2 promotes histone O-GlcNAcylation during gene transcription. Nature 2013; 493:561 - 4; http://dx.doi.org/10.1038/nature11742; PMID: 23222540
- Vella P, Scelfo A, Jammula S, Chiacchiera F, Williams K, Cuomo A, Roberto A, Christensen J, Bonaldi T, Helin K, et al. Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Mol Cell 2013; 49:645 - 56; http://dx.doi.org/10.1016/j.molcel.2012.12.019; PMID: 23352454
- Deplus R, Delatte B, Schwinn MK, Defrance M, Méndez J, Murphy N, Dawson MA, Volkmar M, Putmans P, Calonne E, et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J 2013; 32:645 - 55; http://dx.doi.org/10.1038/emboj.2012.357; PMID: 23353889
- Yu M, Hon GC, Szulwach KE, Song CX, Jin P, Ren B, He C. Tet-assisted bisulfite sequencing of 5-hydroxymethylcytosine. Nat Protoc 2012; 7:2159 - 70; http://dx.doi.org/10.1038/nprot.2012.137; PMID: 23196972
- Sun Z, Terragni J, Borgaro JG, Liu Y, Yu L, Guan S, Wang H, Sun D, Cheng X, Zhu Z, et al. High-resolution enzymatic mapping of genomic 5-hydroxymethylcytosine in mouse embryonic stem cells. Cell Rep 2013; 3:567 - 76; http://dx.doi.org/10.1016/j.celrep.2013.01.001; PMID: 23352666
- McGuffin LJ, Buenavista MT, Roche DB. The ModFOLD4 server for the quality assessment of 3D protein models. Nucleic Acids Res 2013; 41:Web Server issue W368-72; http://dx.doi.org/10.1093/nar/gkt294; PMID: 23620298
- Roche DB, Buenavista MT, Tetchner SJ, McGuffin LJ. The IntFOLD server: an integrated web resource for protein fold recognition, 3D model quality assessment, intrinsic disorder prediction, domain prediction and ligand binding site prediction. Nucleic Acids Res 2011; 39:Web Server issue W171-6; http://dx.doi.org/10.1093/nar/gkr184; PMID: 21459847
- Yang Y, Faraggi E, Zhao H, Zhou Y. Improving protein fold recognition and template-based modeling by employing probabilistic-based matching between predicted one-dimensional structural properties of query and corresponding native properties of templates. Bioinformatics 2011; 27:2076 - 82; http://dx.doi.org/10.1093/bioinformatics/btr350; PMID: 21666270
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 2008; 9:40; http://dx.doi.org/10.1186/1471-2105-9-40; PMID: 18215316
- Zhang Y, Skolnick J. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res 2005; 33:2302 - 9; http://dx.doi.org/10.1093/nar/gki524; PMID: 15849316
- Xu J, Zhang Y. How significant is a protein structure similarity with TM-score = 0.5?. Bioinformatics 2010; 26:889 - 95; http://dx.doi.org/10.1093/bioinformatics/btq066; PMID: 20164152
- Graveley BR, May G, Brooks AN, Carlson JW, Cherbas L, Davis CA, et al. he D. melanogaster transcriptome: modENCODE RNA-Seq data for dissected tissues. FlyBase, personal communication 2011.