Abstract
There is a growing body of evidence that components of the circadian clock are involved in modulation of numerous signaling pathways, and that clock deregulation due to environmental or genetic factors contributes to the development of various pathologies, including cancer. Previous work performed in tissue culture and in in vivo mouse models defined mammalian PERIOD proteins as tumor suppressors, although some experimental inconsistencies (the use of mice on mixed genetic background, lack of sexual discrimination) did not allow a definitive conclusion. To address this issue in a systematic way, we performed a detailed analysis comparing the incidence of tumor development after low-dose ionizing radiation in male and female wild-type, Per1−/−, and Per2−/− mice. We showed that in contrast to previous reports deficiency in either Per1 or Per2 genes by itself does not make mice more tumor-prone; moreover, some of the long-term effects of ionizing radiation in Per2-deficient mice are reminiscent more of accelerated aging rather than tumor-prone phenotype. Our histopathological analysis also revealed significant sexual dimorphism both in the rate of radiation-induced tumorigenesis and in the spectrum of tumors developed, which underscores the importance of using sex-matched experimental groups for in vivo studies. Based on our results, we suggest that the role of PER proteins as bona fide tumor suppressors needs to be reevaluated.
Introduction
It is widely accepted that proper synchronization of various tissues within an organism as well as proper synchronization of an organism with its environment are important factors of an organism's well being. Desynchrony caused by either genetic or environmental factors is associated with development of various pathological conditions, including carcinogenesis. A series of previous epidemiological studies reported a high incidence of tumor formation in shift workersCitation1 and an increased incidence of breast cancer among flight attendants.Citation2,Citation3 Epidemiological data were supported by mouse model studies that showed that disruption of normal rhythmicity either by surgical ablation of the SCN or by chronic exposure to frequent changes in light:dark cycle resulted in faster rates of implanted tumor growth.Citation4 However, molecular mechanisms underlying the clock–cancer connection are still poorly understood. Moreover, recent studies using various circadian mutant mouse models indicate that the relation between carcinogenesis and the circadian clock is more complex than initially proposed.
Hence, none of the circadian mutant mice demonstrate a predisposition to development of spontaneous tumors, and the data on radiation-induced carcinogenesis in mice deficient in different components of the circadian clock remain controversial. Thus, it has been reported that Per2-deficient mice challenged with γ-radiation exhibited a higher rate of tumor incidence as well as more pronounced characteristics of aging (i.e., earlier hair graying).Citation5 The cancer-prone phenotype of Per2-deficient mice was attributed to defects in DNA damage responses associated with changes in the expression patterns of several oncogenes and tumor suppressor genes.Citation5 Independent lines of evidence linking PERIOD proteins and carcinogenesis came from the study of PER expression in human tumor cell lines and tumors. The Per1 expression was changed in primary colorectal tumors,Citation6 in endometrial carcinomas,Citation7 and in non-small cell lung cancer.Citation8 In addition, intratumoral delivery of Per2 inhibited PCNA expression and induced apoptosis in Lewis lung carcinoma tumors, suggesting that the PER2 protein may act as a tumor suppressor.Citation9 At the same time, circadian disruption caused by deficiency of other clock components was not associated with an increase in predisposition for tumor development. Thus, treatment of animals deficient in both Cryptochromes with the DNA-damaging agents does not facilitate carcinogenesis;Citation10 moreover, loss of CRY proteins reduces risk of cancer in highly tumor-prone p53−/− mice.Citation11 In line with these reports, our previous work has demonstrated that ionizing radiation does not increase the rate of tumor formation in Clock/Clock mice.Citation12 Taken together, these data suggest that despite being the essential components of the circadian time-keeping mechanism, each clock protein may be involved in regulation of other pathways in its own unique way. It also raises the question of whether the tumor suppressor function is characteristic to PER proteins, as it has been proposed previouslyCitation5 given some inconsistency both in in vitro and in vivo studies (mixed genetic background of circadian mutant mice used, lack of discrimination between male and female animals, etc.).
To address this controversy, we performed a study involving a large number of wild-type, Per1−/− and Per2−/− male and female mice on pure C57BL/6J genetic background, which all were simultaneously exposed to low dose of total-body irradiation early in life. These animals, together with age-, sex-, and genotype-matched non-irradiated littermates were visually monitored for potential tumor development throughout their lifespan and were analyzed pathohistologically upon completion of the experiment at 86 weeks of age. Here, we show that the rate of spontaneous tumors in non-irradiated mice was very low in all 3 genotypes. Total-body irradiation promoted tumorigenesis in sex- and in gene-specific ways. Thus, WT and Per1−/− female mice appeared to be more tumor-prone than males demonstrating high incidence of ovarian tumors. The rate of ovarian tumors was identical between the 2 genotypes; however, the tumor spectrum was changed. To our surprise, deficiency in either Per1 or Per2 did not make mice more tumor-prone, as incidence of tumors in knockout animals did not differ from sex-matched WT controls. Our data underscore the importance of using standard experimental conditions for in vivo studies and raise the question of whether PER proteins can be considered as bona fide tumor suppressors.
Results
Effects of low-dose of total-body irradiation on lifespan, gross appearance, and body weight of wild-type, Per1−/−, and Per2−/− mice
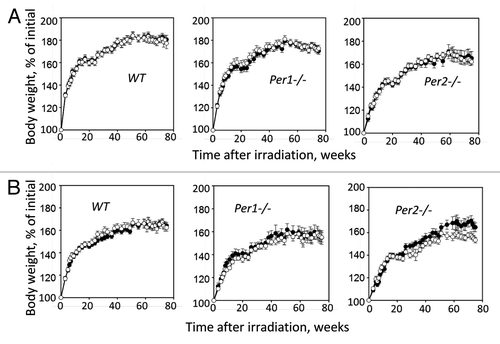
Wild-type, Per1−/−, and Per2−/− untreated and irradiated mice of both genders were monitored at regular intervals for signs and symptoms of illness. As shown in , the survival rate of both male and female mice of all 3 genotypes was very similar, with slightly reduced survival in the irradiated group; however, none of the differences reached statistically significant values (Kaplan–Meier log-rank survival test). provides the summary of the body weight measurements, which were performed biweekly during the entire animals’ lifespan. It shows that irradiation had no effect on total body weight in male mice of all 3 genotypes. In females, only Per2−/− mice demonstrated modest decline in the body weight starting 50 wk after TBI. Evaluation of gross appearance did not reveal any differences between genotypes or sexes. Wild-type, Per1−/−, and Per2−/− start to develop various pathological conditions beginning from 40–50 weeks of age. These pathologies, which include development of kyphosis, eye inflammation, and hair loss, were slightly more pronounced in radiation-treated animals; however they were identical between genotypes and in most cases resemble radiation-induced accelerated aging phenotype of Clock-Δ19 mutant mice.Citation12
Figure 1. The Kalpan–Meier survival curves of male (A) and female (B) WT (black), Per1−/− (blue) and Per2−/− (red) mice after exposure to 4 Gy of TBI. Untreated animals, solid line; irradiated animals, dashed line. Total numbers of animals used in each group are shown on the graph
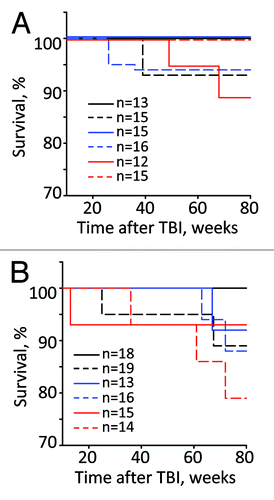
Figure 2. Changes in body weight of WT, Per1−/− and Per2−/− male (A) and female (B) mice monitored through 80 wk after exposure to 4 Gy of total body irradiation. Closed circles, untreated animals; open circles, irradiated animals. Radiation-induced loss of body weight was observed in Per2−/− females only (P = 0.03, Student t test).
Sex- and allele-specific differences in incidence of tumor development in Per-deficient mice
To determine whether TBI increases the incidence of neoplasia in allele-specific and/or gender-specific manner, we performed detailed necropsies on a large group of animals that were sacrificed 80 weeks after exposure to a low dose of TBI. The results of this analysis are summarized in and , which show representative examples of histopathological evaluation of radiation-induced tumors detected in mice of all three genotypes.
Table 1. Summary of histological analysis of various tissues of control and irradiated mice performed at 80 wk after low-dose irradiation (number/% of total)
Figure 3. Representative images of H&E staining of tumors detected in mice of all 3 genotypes. Top row: Lymphoma in irradiated Per1−/− male mouse: large tumor of expansively proliferating uniform neoplastic lymphoid cells encompasses the thymus and mediastinal space (A) and has spread in the white pulp (WP) and partially in the red pulp (RP) of the spleen (B), in the kidney with only single glomeruli (Gl) and tubules(t) remaining visible among the proliferating neoplastic cells(star) (C) and liver (not shown), effacing the normal morphology of these organs. Middle row: Malignant granulosa cell ovarian tumors, found in female mice of all 3 genotypes: monotonous neoplastic cells with clear cytoplasm and rounded or oval nuclei forming follicles, cords, or bands with scanty stroma in between (A); metastasis in mesenteric lymph node with neoplastic cells replacing the normal structure(star) and only remnants of normal lymphocytes (arrow) (B); metastasis in intestinal wall (star) with intact enterocytes(arrow) separating it from the lumen(upper left) (C); metastatic growth(star) in liver leaving thin layer of normal hepatocytes (arrow) (D). Bottom row: Ovarian cystadenoma in irradiated Per1−/− female mouse (A): interconnected spaces lined by a single layer epithelium on mostly acellular underlying stroma; bile duct cystadenoma in liver of irradiated Per2−/− female mouse (B): various in size cysts lined with cuboidal or flattened secreting epithelial cells on mesenchymal stroma or liver parenchyma with normal liver tissue in the right lower corner; Hemangiosarcoma in spleen of irradiated Per1−/− female mouse (C): the normal spleen morphology (upper right) is replaced by variably-sized endothelium-lined vascular spaces (lower right); Hepatoma in liver of irradiated Per2−/− male mouse: rows and plates of well differentiated neoplastic cells (blue star) grow expansively and compress the adjacent normal liver (red arrow).
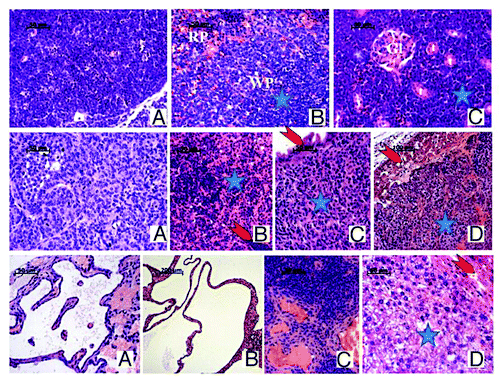
As seen from the data presented in , both male and female non-irradiated WT mice did not show any signs of tumor development by 86-wk-of-age (the time when the experiment was terminated). TBI promoted tumorigenesis in WT mice of both genders: tumors were detected in 5 out of 14 males [36%] and 11 out 19 females [58%]). In males, they were represented by lymphomas and liver cystadenomas (60 and 40%, respectively), while in females the prevailing pathology was the development of ovarian cystadenomas (73%). As expected, statistical analysis confirmed a significant effect of TBI on tumorigenesis (P = 0.039 and P < 0.001 for males and females, respectively, Fisher exact test). No statistically significant sex-specific differences were detected.
When compared with WT animals, mice with a targeted disruption of the Per1 gene showed a modest increase in spontaneous tumorigenesis, which was manifested in males only (4 out 16 mice [25%] developed tumors that were diagnosed as lymphomas and lymphosarcomas [50% each]; however, this difference did not reach statistical significance (P = 0.098, Fisher exact test). Low-dose TBI did not increase rate of tumorigenesis in Per1−/− male mice (only 3 out of 15 [23%] developed tumors represented by 2 cases of lymphoma and 1 case of leukemia). In contrast, TBI significantly increased tumor development in females, both WT (58%) and Per1−/− (63%). These were represented mainly by ovarian granulosa cell tumors (sex-cord stromal malignant tumor) and ovarian cystsadenomas (benign epithelial tumors). Although the incidences of ovarian tumors were similar between the 2 genotypes, deficiency in Per1 resulted in a change in tumor spectrum, with more granulosa cell tumors present in Per1−/− compared with WT females (60% and 9%, respectively). Statistical analysis confirmed the significant effect of TBI on tumor development in Per1−/− females (P < 0.001); the lack of difference between irradiated WT and Per1−/− mice (p = 0.67 and 0.74 for males and females, respectively) and the increase in the incidence of ovarian granulosa cell tumors in Per1−/− when compared with WT females (P = 0.024).
In contrast to our expectations, both male and female Per2−/− mice showed a very low rate of tumorigenesis, both spontaneous and radiation-induced (). The spectrum of tumors was identical to those detected in WT and Per1−/− mice: 1 case of lymphoma and 1 case of liver cystadenoma were detected in irradiated Per2−/− male mice, and 1 liver cystadenoma and 2 granulosa cell tumors were present in irradiated Per2−/− female mice. Taken together, these data indicate that deficiency in PER proteins has no effect of tumor incidence in irradiated mice. It also revealed significant sex-specific differences both in the rate of radiation-induced tumorigenesis and in the spectrum of tumors developed.
Low dose of irradiation induces changes in blood composition in Per2−/−mice reminiscent of the effects of aging.
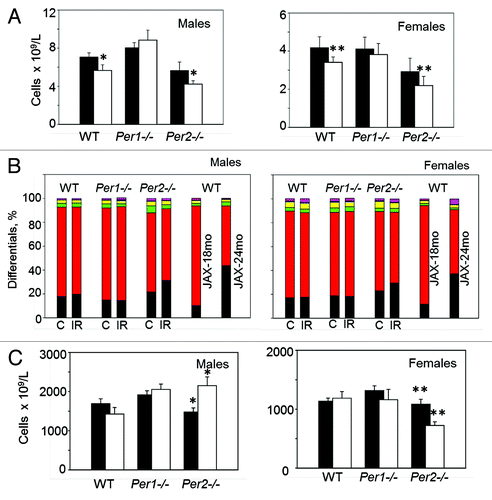
To get additional insight into potential differences between WT and Per-deficient mice in long-term effects of TBI, we performed a total blood cell analysis of control and irradiated animals upon completion of the experiment (at 86 weeks of age). In Per1−/− mice, all basic hematological parameters in control and irradiated animals were identical to those of WT. Similarly, no differences were detected between non-irradiated WT and Per2−/− mice. However, radiation induced changes in several parameters that were specific to a Per2 deficiency. Thus, irradiated mice of both sexes showed a significant reduction in white blood cell counts () and an altered lymphocyte/neutrophil ratio (), which both are characteristic for aging C57BL/6J mice. In addition, irradiated Per2−/− male mice show significant increase in the number of circulating platelets, whereas in females, it was reduced (). These data in combination with our histological analysis and previously reported phenotyping data describing age-dependent increase in the number of platelets (most prominently manifested in males) in C57BL/6J mice (Mouse Phenome Database) suggest that rather than promoting tumorigenesis, TBI of Per2-deficient mice accelerates their aging program.
Figure 4. Whole blood cell analysis of control and irradiated WT, Per1−/− and Per2−/− mice performed 80 wk after exposure to 4 Gy of total body irradiation. Data are presented as mean ± s.d. (A) Per2−/− mice of both sexes show a decrease in the number of white blood cells (*P = 0.02; ** P = 0.03, Student t test); Closed bars, control animals; open bars, irradiated animals. (B) White blood cell composition of control and Irradiated WT, Per1−/− and Per2−/− mice. Each stack in the bar represents cell type percentage: neutrophils are black, lymphocytes are red, monocytes are green, eosinophils are yellow, basophils are blue, and the large unstained cell population is purple; C, control; IR, irradiated. JAX-18mo and JAX-24mo stacks represent cell type percentage in male and female C57BL/6J mice at 18 and 24 mo of age, respectively (Mouse Phenome Database (http://phenome.jax.org) (C) Sex-specific differences in the number of platelets in control and irradiated Per2−/− mice (*P = 0.014; ** P = 0.003, Student t test).
Discussion
Tumor suppressor genes are defined as genes which deregulation (due to mutation, homozygous deletion, epigenetic silencing, haploinsufficiency, etc.) predispose to cancer. Although many functional characteristics of tumor suppressors can be recapitulated in tissue culture models, showing that loss of function results in increased tumor initiation, growth, or progression in vivo is the key proof allowing for defining a gene as a tumor suppressor.Citation13
There are 3 lines of evidence for a tumor-suppressor function of PER proteins. The first one is based on experiments performed in tissue culture, which showed that manipulation of Pers expression affected tumor cell proliferation and their responses to anticancer drugs and radiation. Overexpression of PER2 in the mouse Lewis lung carcinoma (LLC), mammary carcinoma cell line EMT6,Citation14 human K562 leukemia cells,Citation15 and murine sarcoma S-180 cellsCitation16 reduced cellular proliferation and promoted apoptosis. Overexpression of PER2 in human pancreatic cells not only inhibited cell growth, but also sensitized them to cisplatin.Citation17 At the same time, some contradictory reports have demonstrated that LLC and EMT6Citation18 and NIH-3T3Citation19 cells with high levels of PER2 expression show increased resistance to ionizing radiation. Based on these data, it was suggested that PER2 acts as an inhibitor in tumor radiotherapy. Moreover, the implication of PER1 and PER2 in radiation-induced apoptosis as well as direct involvement of PER1 in repair of DNA double-strand brakes via interaction with ATM and CHK2 proteins could not be independently reproduced.Citation20
Another line of evidence is based on numerous observations of deregulation of PER1 and/or PER2 expression in various types of cancer. Thus, expression of Per1, Per2, and Per3 is deregulated in breast cancer tissue.Citation7,Citation21 Per1 (but not Per2) expression was significantly reduced in non-small cell lung, endometrial, pancreatic, prostate, and colorectal cancers.Citation8,Citation22-Citation24 Although potentially important, these observations remain correlative, as it is still not clear whether they represent the cause or the consequence of malignant tumor development. Therefore, demonstration of the tumor-prone phenotype upon deregulation of a certain gene in in vivo mouse model provides the most compelling proof for its tumor suppressor function. However, the data in support of the tumor suppressor function of Pers as well as other circadian genes remain controversial.Citation5,Citation11,Citation12,Citation20,Citation25
Here we used a systematic approach for evaluating the tumor-prone status of mice with deficiencies in either PER1 or PER2 proteins. These 2 lines were maintained on a pure genetic background (C57BL/6J); all mutant and control animals were born and maintained under identical conditions throughout their lifespan; the 3 groups were irradiated at the same time and were monitored using a uniform protocol. Our experimental design and analysis also took into account sex-related differences, an important parameter that is often neglected. As a result, our data revealed prominent sex- and gene-specific variations in radiation-induced tumorigenesis in circadian mutant mice. Most importantly, it indicates that Per deficiency by itself does not make mice more tumor-prone. This was particularly striking in the case of Per2−/− mice, which, in contrast to previous reports,Citation5,Citation25 after exposure to radiation develop similar number of tumors as age- and sex-matched WT animals. One of the potential explanations for the discrepancy is the use of pure genetic background in our study (12 backcross generations to C57BL/6J mice) compared with mixed background of animals used in the original report.Citation5 Previous work performed in highly tumor-prone mouse models (i.e., p53−/− mice) has demonstrated that genetic background has a profound effect on the onset, tissue specificity, morphology, and metastatic potential of tumors.Citation26,Citation27 This argument is somewhat weakened by the apparent use of pure genetic background in the follow up study,Citation25 which, given the coat color variations of the animals used, also raised serious concerns.Citation28 Therefore, at this point, we cannot provide a specific reason for the discrepancy; however, the large number of mice used in our work, the uniform experimental conditions, and the separate analysis performed for male and female animals give us confidence in our conclusions. It is also important to mention that the phenotypic changes observed in Per2-deficient mice after irradiation were reminiscent of the ones developed by Clock-Δ19 mutant mice and described as radiation-induced acceleration of aging program,Citation12 suggesting that PER2 may be defined as an aging suppressor rather than a tumor suppressor protein.
Another interesting observation coming out of our study is the demonstration of the female-specific role of PER1 in tumorigenesis. Histological evaluation of ovarian lesions indicates that although the number of pathological changes in the ovary was identical in WT and Per1−/− females, deficiency in Per1 increases the incidence of granulosa cell tumors (GCT). These tumors comprise a distinct subset of ovarian cancers that account for about 5% of ovarian malignancies in female cancer patientsCitation29 and that are different from ovarian cystadenomas, benign tumors of epithelial origin that were prevailing in irradiated WT females. Although the etiology of GCT remains a debatable issue, there are indications that continuous exposure to estrogen receptor-modulating drugs (routinely used to treat infertility) increases the risk of tumor development.Citation30 In this respect, it is noteworthy that PER1 has been shown to regulate expression of estrogen receptor β (ERβ) via repression of CLOCK/BMAL1-dependent activation of ERβ promoter.Citation31 Consistently, knocking down PER1 expression in a cell-based model led to constant elevation in ERβ expression.Citation31 Thus, the increased incidence of GCT in Per1−/− females may result from constitutively elevated estrogen signaling. Interestingly, a significant gender-specific difference in Per1 expression was observed in colorectal tumors when compared with normal mucosa; Per1 levels were reduced in tissues of female patients only, while no change was detected in tumor samples from males.Citation24
In summary, the data presented here suggest that the role of PER protein as conventional tumor suppressors has to be re-evaluated, and that more research needs to be done to mechanistically dissect the roles of various circadian proteins in tumorigenesis.
Materials and Methods
Animals
Mice with targeted disruption of Per1 or Per2 genes were originally obtained from Dr Weaver.Citation32 Mice were backcrossed to C57BL/6J strain for 12 generations, after which both lines were maintained as homozygous matings. Male and female wild-type (WT), Per1−/−, and Per2−/− mice received 4 Gy of total body irradiation (TBI) at 6 weeks of age (Cs-137 source; 2.5 Gy/min). Animals were weighed biweekly and were closely monitored for any changes in their gross appearance. Animals were sacrificed 80 wk post-irradiation or earlier if they lost more than 20% of their original body weight or displayed any other signs of morbidity. All mice were evaluated for gross pathological changes in major organs, whole-blood cell analysis, and pathohistology of organs that showed gross abnormalities. All animal experiments were approved by the Institutional Animal Care and Use Committee of Roswell Park Cancer Institute.
Total blood cells analysis
Peripheral blood obtained from the retro-orbital sinus was collected into EDTA-treated tubes, and the complete blood counts with differentials were measured using an Advia 120 hematology system (Bayer) and analyzed with the software applications for C57BL/6J mice. All control parameters were within the range previously described for this mouse strain (Justice M. Clinical hematology parameters. MPD:132. Mouse Phenome Database Web Site, the Jackson Laboratory; http://www.jax.org/phenome, 2002).
Histological evaluation
The mice were visually inspected for tumor development and weighed biweekly. For histological evaluation, all tissues were fixed in 10% neutral formalin for 24 h, and then transferred to 70% ethanol. Samples were embedded in paraffin, sectioned and stained with hematoxylin and eosin. Histopathological examination using Zeiss AxioImager A1 with Axiocam MRc digital camera was performed on all tissues with a focus on lymphoid tissues (spleen and thymus) and gross lesions. The guidelines of Bethesda classification were used in determining the diagnosis.Citation33
Statistical analyses
Survival curves were analyzed using the Kaplan–Meier log-rank test; differences in incidents of tumors were evaluated using Fisher exact test; other comparisons were performed using Student t test. P values below 0.05 were considered statistically significant.
Acknowledgments
We thank Dr Weaver for providing Per1−/− and Per2−/− mice and Dr A Sancar for critical reading of the manuscript and helpful comments. This work was supported by NIH grant CA102522 and Roswell Park Alliance Foundation (to MPA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control 2006; 17:489 - 500; http://dx.doi.org/10.1007/s10552-005-9015-4; PMID: 16596302
- Rafnsson V, Sulem P, Tulinius H, Hrafnkelsson J. Breast cancer risk in airline cabin attendants: a nested case-control study in Iceland. Occup Environ Med 2003; 60:807 - 9; http://dx.doi.org/10.1136/oem.60.11.807; PMID: 14573709
- Rafnsson V, Tulinius H, Jónasson JG, Hrafnkelsson J. Risk of breast cancer in female flight attendants: a population-based study (Iceland). Cancer Causes Control 2001; 12:95 - 101; http://dx.doi.org/10.1023/A:1008983416836; PMID: 11246849
- Filipski E, Li XM, Lévi F. Disruption of circadian coordination and malignant growth. Cancer Causes Control 2006; 17:509 - 14; http://dx.doi.org/10.1007/s10552-005-9007-4; PMID: 16596304
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 2002; 111:41 - 50; http://dx.doi.org/10.1016/S0092-8674(02)00961-3; PMID: 12372299
- Krugluger W, Brandstaetter A, Kállay E, Schueller J, Krexner E, Kriwanek S, Bonner E, Cross HS. Regulation of genes of the circadian clock in human colon cancer: reduced period-1 and dihydropyrimidine dehydrogenase transcription correlates in high-grade tumors. Cancer Res 2007; 67:7917 - 22; http://dx.doi.org/10.1158/0008-5472.CAN-07-0133; PMID: 17699798
- Chen ST, Choo KB, Hou MF, Yeh KT, Kuo SJ, Chang JG. Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis 2005; 26:1241 - 6; http://dx.doi.org/10.1093/carcin/bgi075; PMID: 15790588
- Gery S, Komatsu N, Kawamata N, Miller CW, Desmond J, Virk RK, Marchevsky A, Mckenna R, Taguchi H, Koeffler HP. Epigenetic silencing of the candidate tumor suppressor gene Per1 in non-small cell lung cancer. Clin Cancer Res 2007; 13:1399 - 404; http://dx.doi.org/10.1158/1078-0432.CCR-06-1730; PMID: 17332281
- Hua H, Wang Y, Wan C, Liu Y, Zhu B, Wang X, Wang Z, Ding JM. Inhibition of tumorigenesis by intratumoral delivery of the circadian gene mPer2 in C57BL/6 mice. Cancer Gene Ther 2007; 14:815 - 8; http://dx.doi.org/10.1038/sj.cgt.7701061; PMID: 17589433
- Gauger MA, Sancar A. Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res 2005; 65:6828 - 34; http://dx.doi.org/10.1158/0008-5472.CAN-05-1119; PMID: 16061665
- Ozturk N, Lee JH, Gaddameedhi S, Sancar A. Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc Natl Acad Sci U S A 2009; 106:2841 - 6; http://dx.doi.org/10.1073/pnas.0813028106; PMID: 19188586
- Antoch MP, Gorbacheva VY, Vykhovanets O, Toshkov IA, Kondratov RV, Kondratova AA, Lee C, Nikitin AY. Disruption of the circadian clock due to the Clock mutation has discrete effects on aging and carcinogenesis. Cell Cycle 2008; 7:1197 - 204; http://dx.doi.org/10.4161/cc.7.9.5886; PMID: 18418054
- Paige AJ. Redefining tumour suppressor genes: exceptions to the two-hit hypothesis. Cell Mol Life Sci 2003; 60:2147 - 63; http://dx.doi.org/10.1007/s00018-003-3027-6; PMID: 14618262
- Hua H, Wang Y, Wan C, Liu Y, Zhu B, Yang C, Wang X, Wang Z, Cornelissen-Guillaume G, Halberg F. Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Sci 2006; 97:589 - 96; http://dx.doi.org/10.1111/j.1349-7006.2006.00225.x; PMID: 16827798
- Sun CM, Huang SF, Zeng JM, Liu DB, Xiao Q, Tian WJ, Zhu XD, Huang ZG, Feng WL. Per2 inhibits k562 leukemia cell growth in vitro and in vivo through cell cycle arrest and apoptosis induction. Pathol Oncol Res 2010; 16:403 - 11; http://dx.doi.org/10.1007/s12253-009-9227-0; PMID: 19957060
- Miyazaki K, Wakabayashi M, Hara Y, Ishida N. Tumor growth suppression in vivo by overexpression of the circadian component, PER2. Genes Cells 2010; 15:351 - 8; http://dx.doi.org/10.1111/j.1365-2443.2010.01384.x; PMID: 20236181
- Oda A, Katayose Y, Yabuuchi S, Yamamoto K, Mizuma M, Shirasou S, Onogawa T, Ohtsuka H, Yoshida H, Hayashi H, et al. Clock gene mouse period2 overexpression inhibits growth of human pancreatic cancer cells and has synergistic effect with cisplatin. Anticancer Res 2009; 29:1201 - 9; PMID: 19414365
- Zhang J, Zhu B, Liu Y, Jiang Z, Wang Y, Li Y, Hua H, Wang Z. High expression of circadian gene mPer2 diminishes radiosensitivity of tumor cells. Cancer Biother Radiopharm 2008; 23:561 - 70; http://dx.doi.org/10.1089/cbr.2008.0496; PMID: 18999929
- Chang L, Liu YY, Zhu B, Li Y, Hua H, Wang YH, Zhang J, Jiang Z, Wang ZR. High expression of the circadian gene mPer2 diminishes the radiosensitivity of NIH 3T3 cells. Braz J Med Biol Res 2009; 42:882 - 91; http://dx.doi.org/10.1590/S0100-879X2009005000022; PMID: 19787145
- Gaddameedhi S, Reardon JT, Ye R, Ozturk N, Sancar A. Effect of circadian clock mutations on DNA damage response in mammalian cells. Cell Cycle 2012; 11:3481 - 91; http://dx.doi.org/10.4161/cc.21771; PMID: 22918252
- Winter SL, Bosnoyan-Collins L, Pinnaduwage D, Andrulis IL. Expression of the circadian clock genes Per1 and Per2 in sporadic and familial breast tumors. Neoplasia 2007; 9:797 - 800; http://dx.doi.org/10.1593/neo.07595; PMID: 17971899
- Cao Q, Gery S, Dashti A, Yin D, Zhou Y, Gu J, Koeffler HP. A role for the clock gene per1 in prostate cancer. Cancer Res 2009; 69:7619 - 25; http://dx.doi.org/10.1158/0008-5472.CAN-08-4199; PMID: 19752089
- Pogue-Geile KL, Lyons-Weiler J, Whitcomb DC. Molecular overlap of fly circadian rhythms and human pancreatic cancer. Cancer Lett 2006; 243:55 - 7; http://dx.doi.org/10.1016/j.canlet.2005.11.049; PMID: 16451817
- Mostafaie N, Kállay E, Sauerzapf E, Bonner E, Kriwanek S, Cross HS, Huber KR, Krugluger W. Correlated downregulation of estrogen receptor beta and the circadian clock gene Per1 in human colorectal cancer. Mol Carcinog 2009; 48:642 - 7; http://dx.doi.org/10.1002/mc.20510; PMID: 19148895
- Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One 2010; 5:e10995; http://dx.doi.org/10.1371/journal.pone.0010995; PMID: 20539819
- Donehower LA, Harvey M, Vogel H, McArthur MJ, Montgomery CA Jr., Park SH, Thompson T, Ford RJ, Bradley A. Effects of genetic background on tumorigenesis in p53-deficient mice. Mol Carcinog 1995; 14:16 - 22; http://dx.doi.org/10.1002/mc.2940140105; PMID: 7546219
- van Meyel DJ, Sanchez-Sweatman OH, Kerkvliet N, Stitt L, Ramsay DA, Khokha R, Chambers AF, Cairncross JG. Genetic background influences timing, morphology and dissemination of lymphomas in p53-deficient mice. Int J Oncol 1998; 13:917 - 22; PMID: 9772279
- Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY) 2011; 3:479 - 93; PMID: 21566258
- Jamieson S, Fuller PJ. Molecular pathogenesis of granulosa cell tumors of the ovary. Endocr Rev 2012; 33:109 - 44; http://dx.doi.org/10.1210/er.2011-0014; PMID: 22240241
- Willemsen W, Kruitwagen R, Bastiaans B, Hanselaar T, Rolland R. Ovarian stimulation and granulosa-cell tumour. Lancet 1993; 341:986 - 8; http://dx.doi.org/10.1016/0140-6736(93)91071-S; PMID: 8096944
- Cai W, Rambaud J, Teboul M, Masse I, Benoit G, Gustafsson JA, Delaunay F, Laudet V, Pongratz I. Expression levels of estrogen receptor beta are modulated by components of the molecular clock. Mol Cell Biol 2008; 28:784 - 93; http://dx.doi.org/10.1128/MCB.00233-07; PMID: 18039858
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 2001; 30:525 - 36; http://dx.doi.org/10.1016/S0896-6273(01)00302-6; PMID: 11395012
- Morse HC 3rd, Anver MR, Fredrickson TN, Haines DC, Harris AW, Harris NL, Jaffe ES, Kogan SC, MacLennan IC, Pattengale PK, et al, Hematopathology subcommittee of the Mouse Models of Human Cancers Consortium. Bethesda proposals for classification of lymphoid neoplasms in mice. Blood 2002; 100:246 - 58; http://dx.doi.org/10.1182/blood.V100.1.246; PMID: 12070034