Virotherapy, the use of oncolytic viruses for cancer therapy, is an old concept currently making substantial clinical progress. Oncolytic viruses, whose tumor selectivity is genetically engineered or naturally occurring, replicate in and kill cancer cells but not normal tissue, and thus have the potential to amplify themselves in situ and spread throughout the tumor (). Immunovirotherapy takes advantage of the natural inflammatory responses to virus infection and immune responses to oncolytic virus-induced cancer cell death to drive therapeutic anti-tumor immunity and thus act as in situ cancer vaccines and adjuvants.Citation1 The combination of virotherapy with immunotherapy, the active stimulation of effective and durable antitumor immunity, has great promise. Oncolytic herpes simplex virus (oHSV) is particularly suited for immunovirotherapy because of its inherent cytolytic activity and ability to induce robust anti-tumor immunity.Citation2 In addition, the large viral genome can accommodate large and/or multiple therapeutic transgenes that can enhance and modulate immune responses, so-called “armed” oHSV (). One such “armed” oHSV, talimogene laherparepvec (T-Vec, formerly OncoVexGM-CSF), expressing GM-CSF, has shown efficacy in a pivotal phase III melanoma trial (NCT00769704).Citation3 An advantage of “armed” oHSVs is that the transgene is only expressed locally in the tumor, thus avoiding any potential systemic side effects.
Figure 1. Immunovirotherapy using “armed” oHSV. Oncolytic HSV is genetically engineered to selectively replicate in tumor cells (permissive) and not normal cells (non-permissive) through the deletion/inactivation of viral genes necessary for replication in non-tumor cells. Infection of tumor cells with “armed” oHSV results in virus replication, cell death, and expression of the therapeutic transgene (therapeutic product). The therapeutic product (i.e., IL-12 in ref. Citation6) is able to affect normal cells in the tumor microenvironment, like immune cells and endothelial cells, as well as uninfected tumor cells. Death of the infected tumor cells can release tumor antigens and activate the immune system, while newly produced virus can spread in the tumor and infect new tumor cells (virus spread), creating a repetitive cycle.
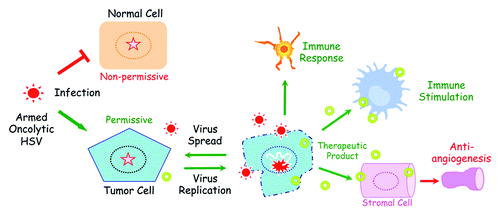
One of the challenges facing preclinical development of oHSV, as well as all oncolytic viruses, is the availability of appropriate tumor models, where safety and efficacy can be evaluated in a setting representative of the clinical situation. Tumors are complex “organs,” consisting of heterogeneous cancer cells and a variety of normal cells, including stromal, vascular, and immune cells. Established tumors typically elucidate a variety of mechanisms to evade immune surveillance and/or antitumor immune responses. We and others have shown that cancer stem cells (CSCs), a subpopulation of tumor cells capable of initiating and sustaining tumor growth with in vitro characteristics of normal adult stem cells, generate xenografts that recapitulate the tumor heterogeneity exhibited by patient tumors and provide representative preclinical tumor models.Citation4,Citation5However, human CSC-derived tumors must be established in immunodeficient mice, precluding evaluation of immunotherapy. An important question is whether CSCs in a syngeneic tumor microenvironment can be therapeutically targeted, including immunologically?
Cheema et al. recently described a new mouse glioblastoma stem cell (GSC)-derived tumor model to test a therapeutic strategy using an “armed” oHSV expressing IL-12.Citation6 This immunocompetent model uses mouse 005 GSCs isolated from gliomas arising in GFAP-cre Tp53+/− transgenic mice after lentivirus transduction of loxP-dependent activated Harvey-Ras and protein kinase B (Akt).Citation7 Implantation of 005 GSCs in the brains of syngeneic C57Bl/6 mice generates tumors with the hallmarks of human glioblastoma: morphologically heterogeneous with multinucleated giant cells and cells expressing stem-cell markers (nestin, prominin), invasive growth into the surrounding brain parenchyma, and angiogenic with aberrant vasculature. In addition, 005 cells are poorly immunogenic (lacking cell surface expression of MHC1, CD40, CD80), and create an immunosuppressive microenvironment in vivo with elevated Tregs, another hallmark of glioblastoma.Citation6 Mice reproducibly develop tumors at the site of implantation with less than 5 × 104 cells within a restricted time frame, making this an excellent and stringent model to test novel therapies.
A third generation oHSV, G47∆, currently in clinical trial for recurrent glioma, was armed with IL-12, a proinflammatory Th1-inducing cytokine involved in innate and adaptive immunity, as well as antiangiogenesis. Among immunomodulatory transgenes examined in oHSV, IL-12 seems to have the greatest potency. Armed G47∆ vectors replicate to some extent in the mouse 005 GSCs in vitro and in GSC-derived intracerebral tumors.Citation6 Expression of IL-12 does not alter virus replication or GSC killing in vitro; however, intratumoral injections of G47∆-mIL12 significantly prolonged survival of tumor-bearing mice compared with the non-transgene control oHSV. Both viruses decreased the number of sphere-forming cells (GSCs) in the tumor, while killing both CD133+ (putative GSCs) and CD133- 005 cells similarly. On the microenvironment side, G47∆-mIL12 reduced neovascularization (anti-angiogenic) and VEGF expression, while inducing angiostatic IP-10 (CXCL10). On the immune side, it reduced the number of immunosuppressive intratumoral Tregs, and its efficacy was abrogated in athymic mice (T cell-deficient), suggesting that IL-12 expression stimulates anti-tumor effector T cells while reducing inhibitory T cells.Citation6 While no significant anti-tumor effect of G47∆-mIL12 was seen in athymic mice, in other studies, G47∆-mIL12 did significantly prolong survival of mice bearing human GSCs, which are more permissive to oHSV replication.Citation8 While promising, G47∆-mIL12 resulted in only 10–20% long-term survivors, leaving room for improvement.
The mouse 005 GSC orthotopic implant tumor model expands the limited number of available immunocompetent glioma models. Importantly, this CSC-derived tumor model permits evaluation of immunotherapeutic strategies, trafficking of immune cells in the tumor, the role of specific genes/cell types using transgenic mice, and immune-mediated effects of oncolytic viruses (beneficial and detrimental). With this model we illustrate the multifaceted activity of G47∆-mIL12, targeting cancer cells via direct oncolysis of GSCs and bulk tumor cells, vasculature through inhibition of angiogenesis, and immune cells by reductions in Tregs and stimulation of T cell-mediated immunity (). Such multifaceted approaches are likely to be critical to successfully treating glioblastoma, which is invariably lethal, as well as other cancers.
References
- Vacchelli E, et al. Oncoimmunology 2013; 2:e24612; http://dx.doi.org/10.4161/onci.24612; PMID: 23894720
- Toda M, et al. Hum Gene Ther 1999; 10:385 - 93; http://dx.doi.org/10.1089/10430349950018832; PMID: 10048391
- Hersey P, et al. J Surg Oncol 2013; http://dx.doi.org/10.1002/jso.23494; PMID: 24301265
- Wakimoto H, et al. Neuro Oncol 2012; 14:132 - 44; http://dx.doi.org/10.1093/neuonc/nor195; PMID: 22067563
- Sugihara E, et al. Int J Cancer 2013; 132:1249 - 59; http://dx.doi.org/10.1002/ijc.27961; PMID: 23180591
- Cheema TA, et al. Proc Natl Acad Sci U S A 2013; 110:12006 - 11; http://dx.doi.org/10.1073/pnas.1307935110; PMID: 23754388
- Marumoto T, et al. Nat Med 2009; 15:110 - 6; http://dx.doi.org/10.1038/nm.1863; PMID: 19122659
- Zhang W, et al. Neoplasia 2013; 15:591 - 9; PMID: 23730207
