Abstract
Aging is not and cannot be programmed. Instead, aging is a continuation of developmental growth, driven by genetic pathways such as mTOR. Ironically, this is often misunderstood as a sort of programmed aging. In contrast, aging is a purposeless quasi-program or, figuratively, a shadow of actual programs.
“The brightest flame casts the darkest shadow.” -George Martin
Introduction
Genes regulate lifespan, in some cases, dramatically.Citation1-Citation17 Pro-aging genes encode signaling pathways such as the insulin/PI3K/TOR pathway that accelerate aging.Citation13-Citation16 These signal-transduction pathways are essential for development, growth, and survival early in life.Citation18 Furthermore, the same signaling pathways drive cellular geroconversion: a conversion from cellular quiescence to senescence.Citation19-Citation40 The same PI3K/TOR pathway is also involved in cancer and other age-related diseases.Citation41-Citation44 The mTOR pathway links development and aging,Citation42 cellular growth and senescence,Citation43 robustness early in life and diseases later in life,Citation44,Citation45 puberty and menopause.Citation46-Citation48 Whereas development and growth are programmed, aging and diseases are not. They are aimless continuations of the program that was not switched off upon its completion. Somehow these notions are confused with programmed aging theory. As discussed,Citation41,Citation49-Citation52 it is only development that is programmed for purpose, aging is not. It is a shadow. Natural selection cannot eliminate the shadow. Nature simply selects for the brightest flame, which in turn casts the darkest shadow.
What Are Programmed Theories of Aging
Aging and its diseases are so orderly that the explanation begs for a program. Like development, aging seems to be programmed.Citation53-Citation57 Programmed theories are thought-provocative and inspiring. They brilliantly illuminate limitations of mainstream theories that aging is a stochastic, random process.Citation53,Citation58 Also, while stochastic aging cannot be prevented,Citation59 the program can be switched off.Citation60-Citation63 This makes programmed theories appealing. But why would nature program aging? It was suggested that aging is beneficial for species and groups.Citation53 There are conditions for group selection in humans, given that human groups had the means to exterminate each other, or using modern terms, to commit genocide. But even group selection cannot select for aging and age-related diseases. In contrast, it should select for robust soldiers, who defend the group from extermination (in human societies and social ants). It was also suggested that organisms undergo programmed death, similar to apoptosis in the multicellular organism.Citation64 Still, aging (at least in humans) is a decades-long process of developing age-related diseases (cancer, hypertension, diabetes, blindness) that terminate life. This is an inefficient way to commit suicide. According to programmed theories, aging prevents overpopulation, speeds up evolution, or benefits young animals, by eliminating old (“less valuable”) animals. But old animals seem less “valuable” precisely because of aging. Thus, aging is programmed to eliminate less valuable animals because of aging. This is a circular reasoning. The only way out from this circle is to suggest that the aging process exists independently of a putative “suicidal program”. But if so, then such a putative program is irrelevant to aging.
Is Aging Programmed in Yeast?
Yeast death in stationary cultures, also known as chronologic senescence, may seem to be programmed.Citation53,Citation65-Citation67 Yeast secretes toxic substances (pheromones, acetic acid, etc.). If “altruistic” yeast die, then other yeast may survive. However, so-called “altruistic” yeast may be less resistant to pH and toxic substances. This simply may be a classic case of survival of the fittest (resistant) yeast. Yeast chronological aging is similar to metabolic self-destruction of human cancer cells.Citation68 In stationary culture, cancer cells acidify the medium with lactic acid. When most cancer cells die, a few cells may survive. Are cancer cells altruistic? In yeast and cancer cell stationary cultures, acid-resistant cells survive. The main difference is that yeast produce acetic acid, whereas cancer cells produce lactic acid.Citation68-Citation72 In yeast, “oncogenic” pathways such as Ras and TOR accelerate chronological senescence.Citation73-Citation75 Inhibitors of the TOR pathway, including rapamycin, decelerate chronological senescence in yeast.Citation75-Citation77 Rapamycin decelerates “yeast-like chronological senescence” in overcrowded cancer cell culture.Citation68 The same signaling pathways (such as TOR) that are involved in chronological senescence in yeast are also involved in metabolic self-destruction of cancer cells.Citation68,Citation73,Citation74,Citation78-Citation81 The same pathways are also involved in cellular geroconversion, organismal aging, and age-related diseases (see ref. Citation68).
Programmed Elements in Non-Programmed (Stochastic) Theories
Programmed theories neither specify nor predict mechanisms of death. Ironically, it was suggested that programmed aging is caused by free radicals.Citation53 And, vice versa, mainstream (stochastic, decay) theories accept special programs (). For example, it was suggested that menopause in women is purposefully programmed to stop reproduction and to raise grandchildren instead.Citation82 Also, it was suggested that the rate of aging is regulated by allocation of energetic resources:Citation83 paradoxically, the more available, the less used.Citation83 It is also thought that aging is programmed in Pacific salmon,Citation84 yet, salmon die from pathologies similar to mammalian age-related diseases. Neither aging and nor age-related diseases in Pacific salmon (or any other animals) are programmed. Aging in Pacific salmon and menopause in women are quasi-programmed.Citation46,Citation85
Table 1. Comparison of 3 groups of theories of aging: programmed, stochastic, and quasi-programmed
Quasi-Programmed Hyperfunction (Aging)
Quasi-programmed aging is not something between “random damage” and “programmed” aging. Instead, quasi-programmed theory is absolutely different from both random damage and programmed theories (). According to quasi-programmed theory,Citation41,Citation42,Citation44,Citation45,Citation49,Citation50,Citation52,Citation86-Citation90 neither aging nor menopause is programmed, they are manifestations of the aging process, which, in turn, is a pseudo-program of developmental growth. There is a mechanistic link between mTOR-driven geroconversion, aging, and age-related pathologies, explaining how cellular hyperfunctions eventually lead to organismal death.Citation41
Quasi-programmed theory predicts mechanisms of aging that are determined by mechanisms of growth, differentiation, and development. There is no need to guess what might be the mechanisms. Aging is a shadow. Its shape is determined by the developmental growth. This can be modeled in cell culture, revealing how growth can be converted to aging.
Quasi-Program of Cellular Senescence
Nutrients, growth factors, hormones, and cytokines all activate nutrient-sensing and growth-promoting signaling pathways such as mTOR (target of rapamycin). mTOR stimulates growth and anabolic metabolism, inhibits autophagy, and increases cellular functions.Citation91-Citation102 Cells grow in size, progress through the cell cycle, and then divide. In the absence of growth factors, normal cells become quiescent: they neither grow nor cycle. In When the cell is stimulated to grow, while the cell cycle is arrested, then the cell becomes senescent (geroconversion).Citation43 mTOR drives growth (program) and geroconversion (quasi-program) (). Also, cellular senescence can be viewed as a continuation of differentiation. The same cytokines that initially cause growth and proliferation then cause cell cycle arrest and differentiation.Citation103-Citation106 During differentiation, cells acquire and amplify specific functions. One example of cellular function is secretion of cytokines, hormones, matrix, enzymes, metabolites, or lipoproteins, depending on cell type. Other examples include contraction of smooth muscle cells, adhesion, and aggregation of platelets as well as oxidative burst of neutrophils.
Figure 1. From cellular growth to hypertrophic senescence (geroconversion). Gerogenic conversion (geroconversion) from cellular growth to cellular aging, when the cell cycle is arrested. Geroconversion is a continuation of growth driven by mTOR and related pathways.
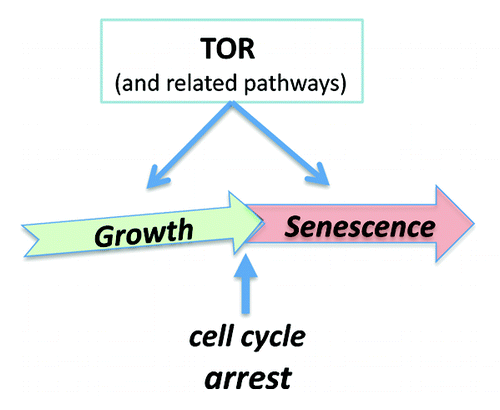
The same intracellular signaling pathways that initially drive proliferation, and then differentiation, also stimulate functions in differentiating cells. Cell senescence-associated hypertrophy and hyper-functions are a continuation of growth ().
From Cellular to Organismal Aging
The most relevant hallmark of cellular aging is hypertrophy/hyperfunctions and compensatory signal resistance, such as insulin resistance. Hyper-functions coupled with signal resistance cause loss of homeostasis, malfunction, organ damage, and death. The link between hyper-functions, including hypertrophy, and diseases has been discussedCitation41,Citation42,Citation50,Citation78,Citation107-Citation110 and will be discussed further (“Aging: From fiction to hyperfunction”, in press).
Quasi-Program of Aging
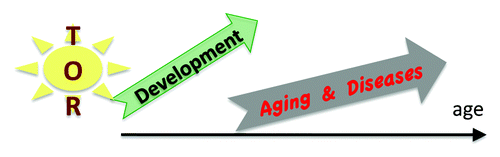
Genetic programs determine developmental growth and the onset of reproduction. When these programs are completed, they are not switched off.
Thus, programs become quasi-programs (). Specific characteristics of quasi-programs of aging and age-related diseases were discussed in detail.Citation41-Citation45,Citation49-Citation52,Citation86-Citation90 The evolutionary theory predicts quasi-programs, like it predicts genes harmful later in life, if they are useful earlier in life.Citation49 I emphasize that the quasi-program does not exist for its own sake: it is a shadow. Aging has no purpose (neither for individuals nor for group), no intention. Nature does not select for quasi-programs. It selects for robust developmental growth. Accelerated aging is the price for robustness.Citation46,Citation50,Citation52,Citation88,Citation111 Although (in some conditions) natural selection works against quasi-programs of aging, it cannot eliminate them without harming development. Genes that drive aging are needed in development. Knockout of PI3K extends the lifespan of C. elegans 10-fold.Citation12 But this comes at a price: prolonged development. Even further, disruption of the mTOR gene leads to post-implantation lethality in mice.Citation112-Citation115 Whereas disruption of S6K1 extends lifespan in mice,Citation14 knockout of both S6K1 and S6K2 causes perinatal lethality.Citation116 In Drosophila, TOR is required for normal growth during larval development.Citation117
Figure 2. From delelopmental growth (program) to aging (shadow). Quasi-programmed aging is driven by over-activation of signal-transduction pathways such as TOR and exacerbation of normal cellular functions, which become harmful (hyper-function), leading to alterations of homeostasis, malfunctions, diseases, and organ damage.
The Utility of the Model
Mechanisms of aging are not arbitrary but determined by mechanisms of development and growth. Since development and growth are relatively well understood, we can interpolate this knowledge to studying aging. For example, it is known that mTOR drives cellular mass growth. This predicts that p53 and hypoxia, which inhibit mTOR and cellular mass growth, will suppress geroconversion despite causing cell cycle arrest.Citation25,Citation26,Citation118-Citation120 Thus, like other tumor suppressors,Citation43 p53 and hypoxia may play a dual role in aging.Citation121-Citation128 The map of growth-promoting signaling network can be interpolated to aging. Gerogenes (insulin receptor, PI-3K, Akt, mTOR) and gerosuppressors (PTEN, TSC, AMPK) form a network, which (in analogy with the periodic “Mendeleev” table) predicts the effect of a particular gene on aging and diseases.Citation129 Basically, genes that activate the mTOR pathway are gerogenes, and those that antagonize the pathway are gerosuppressors.Citation43,Citation129 As another example, developmental trends, such as an increase in blood pressure, near vision point, and FSH levels (all necessary for development and reproductive functions) cause hypertension, presbyopia, and menopause, respectively, later in life.Citation89 Many predictions of the quasi-programmed aging modelCitation42 were confirmed by 2010,Citation87 including the prediction that rapamycin will extend lifespan in mice.Citation130 Numerous recent publications further illuminate the role of the mTOR pathway (and related pathways) in aging.Citation35,Citation131-Citation168
If used properly, rapamycin improves immunity and decreases infections and their complications.Citation148,Citation169,Citation170 Under certain conditions, rapamycin can exert immunostimulatory effects, boosting T-cell responses in the face of pathogen infections and vaccines.Citation170,Citation171 Rapamycin may improve response against pathogens but prevent transplant rejection.Citation172,Citation173
Conclusion
The essence of quasi-program was discussed previously.Citation42,Citation89 Here I addressed a misunderstanding that a quasi-program is a sort of a program. It is not (). Whereas the growth of the body is programmed, the emergence of the shadow is not. Natural selection cannot eliminate the shadow without hurting the “body”. As a case in point, mTOR knockout is lethal in embryogenesis. However, pharmacologic interventions can be started in post-development, thus extending healthy lifespan. MTOR-driven quasi-program can be suppressed pharmacologically.Citation174 And this is what is actually important. After all, according to Oscar Wilde, “What men call the shadow of the body is not the shadow of the body, but is the body of the soul.”
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature 1993; 366:461 - 4; http://dx.doi.org/10.1038/366461a0; PMID: 8247153
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature 2000; 408:255 - 62; http://dx.doi.org/10.1038/35041700; PMID: 11089983
- Partridge L, Gems D. Mechanisms of ageing: public or private?. Nat Rev Genet 2002; 3:165 - 75; http://dx.doi.org/10.1038/nrg753; PMID: 11972154
- Hekimi S, Guarente L. Genetics and the specificity of the aging process. Science 2003; 299:1351 - 4; http://dx.doi.org/10.1126/science.1082358; PMID: 12610295
- Sinclair DA, Guarente L. Unlocking the secrets of longevity genes. Sci Am 2006; 294:48 - 51, 54-7; http://dx.doi.org/10.1038/scientificamerican0306-48; PMID: 16502611
- Gardner MP, Gems D, Viney ME. Extraordinary plasticity in aging in Strongyloides ratti implies a gene-regulatory mechanism of lifespan evolution. Aging Cell 2006; 5:315 - 23; http://dx.doi.org/10.1111/j.1474-9726.2006.00226.x; PMID: 16913877
- Kenyon CJ. The genetics of ageing. Nature 2010; 464:504 - 12; http://dx.doi.org/10.1038/nature08980; PMID: 20336132
- Kaeberlein M. Longevity and aging. F1000Prime Rep 2013; 5:5; http://dx.doi.org/10.12703/P5-5; PMID: 23513177
- Gladyshev VN, Zhang G, Wang J. The naked mole rat genome: understanding aging through genome analysis. Aging (Albany NY) 2011; 3:1124; PMID: 22199030
- Bartke A, Coschigano K, Kopchick J, Chandrashekar V, Mattison J, Kinney B, Hauck S. Genes that prolong life: relationships of growth hormone and growth to aging and lifespan. J Gerontol A Biol Sci Med Sci 2001; 56:B340 - 9; http://dx.doi.org/10.1093/gerona/56.8.B340; PMID: 11487592
- Bartke A. Insulin and aging. Cell Cycle 2008; 7:3338 - 43; http://dx.doi.org/10.4161/cc.7.21.7012; PMID: 18948730
- Ayyadevara S, Alla R, Thaden JJ, Shmookler Reis RJ. Remarkable longevity and stress resistance of nematode PI3K-null mutants. Aging Cell 2008; 7:13 - 22; http://dx.doi.org/10.1111/j.1474-9726.2007.00348.x; PMID: 17996009
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 2004; 14:885 - 90; http://dx.doi.org/10.1016/j.cub.2004.03.059; PMID: 15186745
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian lifespan. Science 2009; 326:140 - 4; http://dx.doi.org/10.1126/science.1177221; PMID: 19797661
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature 2013; 493:338 - 45; http://dx.doi.org/10.1038/nature11861; PMID: 23325216
- Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep 2013; 4:913 - 20; http://dx.doi.org/10.1016/j.celrep.2013.07.030; PMID: 23994476
- Hoffmann J, Romey R, Fink C, Yong L, Roeder T. Overexpression of Sir2 in the adult fat body is sufficient to extend lifespan of male and female Drosophila. Aging (Albany NY) 2013; 5:315 - 27; PMID: 23765091
- Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging (Albany NY) 2009; 1:357 - 62; PMID: 20157523
- Blagosklonny MV. Cell senescence and hypermitogenic arrest. EMBO Rep 2003; 4:358 - 62; http://dx.doi.org/10.1038/sj.embor.embor806; PMID: 12671679
- Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle 2008; 7:3355 - 61; http://dx.doi.org/10.4161/cc.7.21.6919; PMID: 18948731
- Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle 2009; 8:1888 - 95; http://dx.doi.org/10.4161/cc.8.12.8606; PMID: 19471117
- Demidenko ZN, Shtutman M, Blagosklonny MV. Pharmacologic inhibition of MEK and PI-3K converges on the mTOR/S6 pathway to decelerate cellular senescence. Cell Cycle 2009; 8:1896 - 900; http://dx.doi.org/10.4161/cc.8.12.8809; PMID: 19478560
- Demidenko ZN, Blagosklonny MV. Quantifying pharmacologic suppression of cellular senescence: prevention of cellular hypertrophy versus preservation of proliferative potential. Aging (Albany NY) 2009; 1:1008 - 16; PMID: 20157583
- Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging (Albany NY) 2010; 2:344 - 52; PMID: 20606252
- Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci U S A 2010; 107:9660 - 4; http://dx.doi.org/10.1073/pnas.1002298107; PMID: 20457898
- Leontieva OV, Natarajan V, Demidenko ZN, Burdelya LG, Gudkov AV, Blagosklonny MV. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc Natl Acad Sci U S A 2012; 109:13314 - 8; http://dx.doi.org/10.1073/pnas.1205690109; PMID: 22847439
- Pospelova TV, Demidenko ZN, Bukreeva EI, Pospelov VA, Gudkov AV, Blagosklonny MV. Pseudo-DNA damage response in senescent cells. Cell Cycle 2009; 8:4112 - 8; http://dx.doi.org/10.4161/cc.8.24.10215; PMID: 19946210
- Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany NY) 2010; 2:924 - 35; PMID: 21212465
- Leontieva OV, Demidenko ZN, Blagosklonny MV. MEK drives cyclin D1 hyperelevation during geroconversion. Cell Death Differ 2013; 20:1241 - 9; http://dx.doi.org/10.1038/cdd.2013.86; PMID: 23852369
- Leontieva OV, Blagosklonny MV. CDK4/6-inhibiting drug substitutes for p21 and p16 in senescence: duration of cell cycle arrest and MTOR activity determine geroconversion. Cell Cycle 2013; 12:3063 - 9; http://dx.doi.org/10.4161/cc.26130; PMID: 23974099
- Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle 2012; 11:2391 - 401; http://dx.doi.org/10.4161/cc.20683; PMID: 22627671
- Darzynkiewicz Z. Forever young, slim and fit: rapamycin to the rescue. Cell Cycle 2009; 8:1820 - 1; http://dx.doi.org/10.4161/cc.8.12.8967; PMID: 19471126
- Romanov VS, Abramova MV, Svetlikova SB, Bykova TV, Zubova SG, Aksenov ND, Fornace AJ Jr., Pospelova TV, Pospelov VA. p21(Waf1) is required for cellular senescence but not for cell cycle arrest induced by the HDAC inhibitor sodium butyrate. Cell Cycle 2010; 9:3945 - 55; http://dx.doi.org/10.4161/cc.9.19.13160; PMID: 20935470
- Zhao H, Halicka HD, Li J, Darzynkiewicz Z. Berberine suppresses gero-conversion from cell cycle arrest to senescence. Aging (Albany NY) 2013; 5:623 - 36; PMID: 23974852
- Halicka HD, Zhao H, Li J, Lee YS, Hsieh TC, Wu JM, Darzynkiewicz Z. Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling. Aging (Albany NY) 2012; 4:952 - 65; PMID: 23363784
- Chen C, Liu Y, Liu Y, Zheng P. The axis of mTOR-mitochondria-ROS and stemness of the hematopoietic stem cells. Cell Cycle 2009; 8:1158 - 60; http://dx.doi.org/10.4161/cc.8.8.8139; PMID: 19270502
- Gan B, DePinho RA. mTORC1 signaling governs hematopoietic stem cell quiescence. Cell Cycle 2009; 8:1003 - 6; http://dx.doi.org/10.4161/cc.8.7.8045; PMID: 19270523
- Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, Hong S, Berry LS, Reichelt S, Ferreira M, et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 2011; 332:966 - 70; http://dx.doi.org/10.1126/science.1205407; PMID: 21512002
- Young AR, Narita M, Narita M. Spatio-temporal association between mTOR and autophagy during cellular senescence. Autophagy 2011; 7:1387 - 8; http://dx.doi.org/10.4161/auto.7.11.17348; PMID: 21799306
- Fujii S, Hara H, Araya J, Takasaka N, Kojima J, Ito S, Minagawa S, Yumino Y, Ishikawa T, Numata T, et al. Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. Oncoimmunology 2012; 1:630 - 41; http://dx.doi.org/10.4161/onci.20297; PMID: 22934255
- Blagosklonny MV. Answering the ultimate question “what is the proximal cause of aging?”. Aging (Albany NY) 2012; 4:861 - 77; PMID: 23425777
- Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle 2006; 5:2087 - 102; http://dx.doi.org/10.4161/cc.5.18.3288; PMID: 17012837
- Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012; 4:159 - 65; PMID: 22394614
- Blagosklonny MV. TOR-driven aging: speeding car without brakes. Cell Cycle 2009; 8:4055 - 9; http://dx.doi.org/10.4161/cc.8.24.10310; PMID: 19923900
- Blagosklonny MV. Big mice die young but large animals live longer. Aging (Albany NY) 2013; 5:227 - 33; PMID: 23603822
- Blagosklonny MV. Why men age faster but reproduce longer than women: mTOR and evolutionary perspectives. Aging (Albany NY) 2010; 2:265 - 73; PMID: 20519781
- Adhikari D, Liu K. mTOR signaling in the control of activation of primordial follicles. Cell Cycle 2010; 9:1673 - 4; http://dx.doi.org/10.4161/cc.9.9.11626; PMID: 20404510
- Luo LL, Xu JJ, Fu YC. Rapamycin prolongs female reproductive lifespan. Cell Cycle 2013; 12:3353 - 4; http://dx.doi.org/10.4161/cc.26578; PMID: 24091532
- Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle 2010; 9:3151 - 6; http://dx.doi.org/10.4161/cc.9.16.13120; PMID: 20724817
- Blagosklonny MV. Why the disposable soma theory cannot explain why women live longer and why we age. Aging (Albany NY) 2010; 2:884 - 7; PMID: 21191147
- Blagosklonny MV. Hormesis does not make sense except in the light of TOR-driven aging. Aging (Albany NY) 2011; 3:1051 - 62; PMID: 22166724
- Blagosklonny MV. MTOR-driven quasi-programmed aging as a disposable soma theory: blind watchmaker vs. intelligent designer. Cell Cycle 2013; 12:1842 - 7; http://dx.doi.org/10.4161/cc.25062; PMID: 23708516
- Longo VD, Mitteldorf J, Skulachev VP. Programmed and altruistic ageing. Nat Rev Genet 2005; 6:866 - 72; http://dx.doi.org/10.1038/nrg1706; PMID: 16304601
- Bredesen DE. The non-existent aging program: how does it work?. Aging Cell 2004; 3:255 - 9; http://dx.doi.org/10.1111/j.1474-9728.2004.00121.x; PMID: 15379848
- Prinzinger R. Programmed ageing: the theory of maximal metabolic scope. How does the biological clock tick?. EMBO Rep 2005; 6:S14 - 9; http://dx.doi.org/10.1038/sj.embor.7400425; PMID: 15995655
- de Magalhães JP. Programmatic features of aging originating in development: aging mechanisms beyond molecular damage?. FASEB J 2012; 26:4821 - 6; http://dx.doi.org/10.1096/fj.12-210872; PMID: 22964300
- de Magalhães JP, Church GM. Cells discover fire: employing reactive oxygen species in development and consequences for aging. Exp Gerontol 2006; 41:1 - 10; http://dx.doi.org/10.1016/j.exger.2005.09.002; PMID: 16226003
- Mitteldorf J. Can experiments on caloric restriction be reconciled with the disposable soma theory for the evolution of senescence?. [ discussion 1906] Evolution 2001; 55:1902 - 5, discussion 1906; PMID: 11681746
- Hayflick L. “Anti-aging” is an oxymoron. J Gerontol A Biol Sci Med Sci 2004; 59:B573 - 8; http://dx.doi.org/10.1093/gerona/59.6.B573; PMID: 15215267
- Skulachev VP, Longo VD. Aging as a mitochondria-mediated atavistic program: can aging be switched off?. Ann N Y Acad Sci 2005; 1057:145 - 64; http://dx.doi.org/10.1196/annals.1356.009; PMID: 16399892
- Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG, et al. An attempt to prevent senescence: a mitochondrial approach. Biochim Biophys Acta 2009; 1787:437 - 61; http://dx.doi.org/10.1016/j.bbabio.2008.12.008; PMID: 19159610
- Skulachev VP. SkQ1 treatment and food restriction--two ways to retard an aging program of organisms. Aging (Albany NY) 2011; 3:1045 - 50; PMID: 22170754
- Skulachev VP. Aging as a particular case of phenoptosis, the programmed death of an organism (a response to Kirkwood and Melov “On the programmed/non-programmed nature of ageing within the life history”). Aging (Albany NY) 2011; 3:1120 - 3; PMID: 22146104
- Skulachev VP. Programmed death phenomena: from organelle to organism. Ann N Y Acad Sci 2002; 959:214 - 37; http://dx.doi.org/10.1111/j.1749-6632.2002.tb02095.x; PMID: 11976198
- Herker E, Jungwirth H, Lehmann KA, Maldener C, Fröhlich KU, Wissing S, Büttner S, Fehr M, Sigrist S, Madeo F. Chronological aging leads to apoptosis in yeast. J Cell Biol 2004; 164:501 - 7; http://dx.doi.org/10.1083/jcb.200310014; PMID: 14970189
- Fabrizio P, Battistella L, Vardavas R, Gattazzo C, Liou LL, Diaspro A, Dossen JW, Gralla EB, Longo VD. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J Cell Biol 2004; 166:1055 - 67; http://dx.doi.org/10.1083/jcb.200404002; PMID: 15452146
- Fabrizio P, Longo VD. Chronological aging-induced apoptosis in yeast. Biochim Biophys Acta 2008; 1783:1280 - 5; http://dx.doi.org/10.1016/j.bbamcr.2008.03.017; PMID: 18445486
- Leontieva OV, Blagosklonny MV. Yeast-like chronological senescence in mammalian cells: phenomenon, mechanism and pharmacological suppression. Aging (Albany NY) 2011; 3:1078 - 91; PMID: 22156391
- Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. A molecular mechanism of chronological aging in yeast. Cell Cycle 2009; 8:1256 - 70; http://dx.doi.org/10.4161/cc.8.8.8287; PMID: 19305133
- Burtner CR, Murakami CJ, Kaeberlein M. A genomic approach to yeast chronological aging. Methods Mol Biol 2009; 548:101 - 14; http://dx.doi.org/10.1007/978-1-59745-540-4_6; PMID: 19521821
- Kaeberlein M. Lessons on longevity from budding yeast. Nature 2010; 464:513 - 9; http://dx.doi.org/10.1038/nature08981; PMID: 20336133
- Fabrizio P, Wei M. Conserved role of medium acidification in chronological senescence of yeast and mammalian cells. Aging (Albany NY) 2011; 3:1127 - 9; PMID: 22184281
- Longo VD. Ras: the other pro-aging pathway. Sci Aging Knowledge Environ 2004; 2004:pe36; http://dx.doi.org/10.1126/sageke.2004.39.pe36; PMID: 15456908
- Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological lifespan via increased respiration and upregulation of mitochondrial gene expression. Cell Metab 2007; 5:265 - 77; http://dx.doi.org/10.1016/j.cmet.2007.02.009; PMID: 17403371
- Powers RW 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological lifespan in yeast by decreased TOR pathway signaling. Genes Dev 2006; 20:174 - 84; http://dx.doi.org/10.1101/gad.1381406; PMID: 16418483
- Alvers AL, Wood MS, Hu D, Kaywell AC, Dunn WA Jr., Aris JP. Autophagy is required for extension of yeast chronological lifespan by rapamycin. Autophagy 2009; 5:847 - 9; PMID: 19458476
- Pan Y, Shadel GS. Extension of chronological lifespan by reduced TOR signaling requires down-regulation of Sch9p and involves increased mitochondrial OXPHOS complex density. Aging (Albany NY) 2009; 1:131 - 45; PMID: 20157595
- Blagosklonny MV. Molecular damage in cancer: an argument for mTOR-driven aging. Aging (Albany NY) 2011; 3:1130 - 41; PMID: 22246147
- Kaeberlein M, Hu D, Kerr EO, Tsuchiya M, Westman EA, Dang N, Fields S, Kennedy BK. Increased lifespan due to calorie restriction in respiratory-deficient yeast. PLoS Genet 2005; 1:e69; http://dx.doi.org/10.1371/journal.pgen.0010069; PMID: 16311627
- Powers T. TOR signaling and S6 kinase 1: Yeast catches up. Cell Metab 2007; 6:1 - 2; http://dx.doi.org/10.1016/j.cmet.2007.06.009; PMID: 17618850
- Kennedy BK, Steffen KK, Kaeberlein M. Ruminations on dietary restriction and aging. Cell Mol Life Sci 2007; 64:1323 - 8; http://dx.doi.org/10.1007/s00018-007-6470-y; PMID: 17396225
- Shanley DP, Kirkwood TB. Evolution of the human menopause. Bioessays 2001; 23:282 - 7; http://dx.doi.org/10.1002/1521-1878(200103)23:3<282::AID-BIES1038>3.0.CO;2-9; PMID: 11223885
- Kirkwood TB, Austad SN. Why do we age?. Nature 2000; 408:233 - 8; http://dx.doi.org/10.1038/35041682; PMID: 11089980
- Austad SN. Is aging programed?. Aging Cell 2004; 3:249 - 51; http://dx.doi.org/10.1111/j.1474-9728.2004.00112.x; PMID: 15379846
- Blagosklonny MV. Paradoxes of aging. Cell Cycle 2007; 6:2997 - 3003; http://dx.doi.org/10.4161/cc.6.24.5124; PMID: 18156807
- Blagosklonny MV. Program-like aging and mitochondria: instead of random damage by free radicals. J Cell Biochem 2007; 102:1389 - 99; http://dx.doi.org/10.1002/jcb.21602; PMID: 17975792
- Blagosklonny MV. Rapamycin and quasi-programmed aging: four years later. Cell Cycle 2010; 9:1859 - 62; http://dx.doi.org/10.4161/cc.9.10.11872; PMID: 20436272
- Blagosklonny MV. Why human lifespan is rapidly increasing: solving “longevity riddle” with “revealed-slow-aging” hypothesis. Aging (Albany NY) 2010; 2:177 - 82; PMID: 20404395
- Blagosklonny MV. Rapamycin extends life- and health span because it slows aging. Aging (Albany NY) 2013; 5:592 - 8; PMID: 23934728
- Blagosklonny MV. M(o)TOR of aging: MTOR as a universal molecular hypothalamus. Aging (Albany NY) 2013; 5:490 - 4; PMID: 23872658
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006; 124:471 - 84; http://dx.doi.org/10.1016/j.cell.2006.01.016; PMID: 16469695
- Hands SL, Proud CG, Wyttenbach A. mTOR’s role in ageing: protein synthesis or autophagy?. Aging (Albany NY) 2009; 1:586 - 97; PMID: 20157541
- Yan L, Mieulet V, Lamb RF. Nutrient regulation of mTORC1 and cell growth. Cell Cycle 2010; 9:2473 - 4; http://dx.doi.org/10.4161/cc.9.13.12124; PMID: 20543582
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 2010; 40:310 - 22; http://dx.doi.org/10.1016/j.molcel.2010.09.026; PMID: 20965424
- Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev 2013; 23:53 - 62; http://dx.doi.org/10.1016/j.gde.2012.12.005; PMID: 23317514
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell 2007; 12:487 - 502; http://dx.doi.org/10.1016/j.devcel.2007.03.020; PMID: 17419990
- Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 2011; 189:1177 - 201; http://dx.doi.org/10.1534/genetics.111.133363; PMID: 22174183
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011; 12:21 - 35; http://dx.doi.org/10.1038/nrm3025; PMID: 21157483
- Kim E, Guan KL. RAG GTPases in nutrient-mediated TOR signaling pathway. Cell Cycle 2009; 8:1014 - 8; http://dx.doi.org/10.4161/cc.8.7.8124; PMID: 19270521
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153:1194 - 217; http://dx.doi.org/10.1016/j.cell.2013.05.039; PMID: 23746838
- Hildebrand DG, Lehle S, Borst A, Haferkamp S, Essmann F, Schulze-Osthoff K. α-Fucosidase as a novel convenient biomarker for cellular senescence. Cell Cycle 2013; 12:1922 - 7; http://dx.doi.org/10.4161/cc.24944; PMID: 23673343
- Völkers M, Sussman M. mTOR/PRAS40 interaction: Hypertrophy or proliferation. Cell Cycle 2013; 12; In press http://dx.doi.org/10.4161/cc.26822; PMID: 24131922
- Ogawa M. Hemopoietic stem cells: stochastic differentiation and humoral control of proliferation. Environ Health Perspect 1989; 80:199 - 207; http://dx.doi.org/10.1289/ehp.8980199; PMID: 2647480
- Watari K, Tojo A, Nagamura-Inoue T, Matsuoka M, Irie S, Tani K, Yamada Y, Asano S. Hyperfunction of neutrophils in a patient with BCR/ABL negative chronic myeloid leukemia: a case report with in vitro studies. Cancer 2000; 89:551 - 60; http://dx.doi.org/10.1002/1097-0142(20000801)89:3<551::AID-CNCR10>3.0.CO;2-E; PMID: 10931454
- Metcalf D. Implications of the polyfunctionality of hemopoietic regulators. Stem Cells 1994; 12:Suppl 1 259 - 75; http://dx.doi.org/10.1002/stem.5530120722; PMID: 7696965
- Baumann MA, Paul CC, Lemley-Gillespie S, Oyster M, Gomez-Cambronero J. Modulation of MEK activity during G-CSF signaling alters proliferative versus differentiative balancing. Am J Hematol 2001; 68:99 - 105; http://dx.doi.org/10.1002/ajh.1160; PMID: 11559949
- Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. Am J Pathol 2012; 181:1142 - 6; http://dx.doi.org/10.1016/j.ajpath.2012.06.024; PMID: 22841821
- Blagosklonny MV. Aging-suppressants: cellular senescence (hyperactivation) and its pharmacologic deceleration. Cell Cycle 2009; 8:1883 - 7; http://dx.doi.org/10.4161/cc.8.12.8815; PMID: 19448395
- Blagosklonny MV. Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans). Cell Cycle 2010; 9:683 - 8; http://dx.doi.org/10.4161/cc.9.4.10766; PMID: 20139716
- Blagosklonny MV. Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging (Albany NY) 2012; 4:350 - 8; PMID: 22683661
- Blagosklonny MV. Increasing healthy lifespan by suppressing aging in our lifetime: preliminary proposal. Cell Cycle 2010; 9:4788 - 94; http://dx.doi.org/10.4161/cc.9.24.14360; PMID: 21150328
- Hentges KE, Sirry B, Gingeras AC, Sarbassov D, Sonenberg N, Sabatini D, Peterson AS. FRAP/mTOR is required for proliferation and patterning during embryonic development in the mouse. Proc Natl Acad Sci U S A 2001; 98:13796 - 801; http://dx.doi.org/10.1073/pnas.241184198; PMID: 11707573
- Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol 2004; 24:9508 - 16; http://dx.doi.org/10.1128/MCB.24.21.9508-9516.2004; PMID: 15485918
- Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol 2004; 24:6710 - 8; http://dx.doi.org/10.1128/MCB.24.15.6710-6718.2004; PMID: 15254238
- Shor B, Cavender D, Harris C. A kinase-dead knock-in mutation in mTOR leads to early embryonic lethality and is dispensable for the immune system in heterozygous mice. BMC Immunol 2009; 10:28; http://dx.doi.org/10.1186/1471-2172-10-28; PMID: 19457267
- Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol 2004; 24:3112 - 24; http://dx.doi.org/10.1128/MCB.24.8.3112-3124.2004; PMID: 15060135
- Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev 2000; 14:2712 - 24; http://dx.doi.org/10.1101/gad.835000; PMID: 11069888
- Santoro R, Blandino G. p53: The pivot between cell cycle arrest and senescence. Cell Cycle 2010; 9:4262 - 3; http://dx.doi.org/10.4161/cc.9.21.13853; PMID: 21057199
- Loayza-Puch F, Drost J, Rooijers K, Lopes R, Elkon R, Agami R. p53 induces transcriptional and translational programs to suppress cell proliferation and growth. Genome Biol 2013; 14:R32; http://dx.doi.org/10.1186/gb-2013-14-4-r32; PMID: 23594524
- Serrano M. Shifting senescence into quiescence by turning up p53. Cell Cycle 2010; 9:4256 - 7; http://dx.doi.org/10.4161/cc.9.21.13785; PMID: 20980826
- Feng Z, Hu W, Rajagopal G, Levine AJ. The tumor suppressor p53: cancer and aging. Cell Cycle 2008; 7:842 - 7; http://dx.doi.org/10.4161/cc.7.7.5657; PMID: 18414039
- Roemer K. Are the conspicuous interdependences of fecundity, longevity and cognitive abilities in humans caused in part by p53?. Cell Cycle 2010; 9:3438 - 41; http://dx.doi.org/10.4161/cc.9.17.13001; PMID: 20814241
- Bauer JH, Helfand SL. Sir2 and longevity: the p53 connection. Cell Cycle 2009; 8:1821; http://dx.doi.org/10.4161/cc.8.12.9010; PMID: 19471127
- Hasty P, Sharp ZD, Curiel TJ, Campisi J. mTORC1 and p53: clash of the gods?. Cell Cycle 2013; 12:20 - 5; http://dx.doi.org/10.4161/cc.22912; PMID: 23255104
- Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, Flores JM, Viña J, Blasco MA, Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 2007; 448:375 - 9; http://dx.doi.org/10.1038/nature05949; PMID: 17637672
- Berkers CR, Maddocks OD, Cheung EC, Mor I, Vousden KH. Metabolic Regulation by p53 Family Members. Cell Metab 2013; 18:617 - 33; http://dx.doi.org/10.1016/j.cmet.2013.06.019; PMID: 23954639
- Tower J. The genetic architecture of aging: sexual antagonistic pleiotropy of p53 and foxo. Cell Cycle 2010; 9:3840 - 1; http://dx.doi.org/10.4161/cc.9.19.13464; PMID: 20935483
- Kaeberlein M, Kapahi P. The hypoxic response and aging. Cell Cycle 2009; 8:2324; http://dx.doi.org/10.4161/cc.8.15.9126; PMID: 19633411
- Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug Discov Today 2007; 12:218 - 24; http://dx.doi.org/10.1016/j.drudis.2007.01.004; PMID: 17331886
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009; 460:392 - 5; PMID: 19587680
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. Rapamycin, but not resveratrol or simvastatin, extends lifespan of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 2011; 66:191 - 201; http://dx.doi.org/10.1093/gerona/glq178; PMID: 20974732
- Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell 2012; 11:326 - 35; http://dx.doi.org/10.1111/j.1474-9726.2011.00791.x; PMID: 22212527
- Zheng XF. Chemoprevention of age-related macular regeneration (AMD) with rapamycin. Aging (Albany NY) 2012; 4:375 - 6; PMID: 22796653
- Khanna A, Kapahi P. Rapamycin: killing two birds with one stone. Aging (Albany NY) 2011; 3:1043 - 4; PMID: 22170738
- Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB, Austad SN, Strong R, Richardson A, Hart MJ, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience 2012; 223:102 - 13; http://dx.doi.org/10.1016/j.neuroscience.2012.06.054; PMID: 22750207
- Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, et al. If started early in life, metformin treatment increases lifespan and postpones tumors in female SHR mice. Aging (Albany NY) 2011; 3:148 - 57; PMID: 21386129
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. Rapamycin slows aging in mice. Aging Cell 2012; 11:675 - 82; http://dx.doi.org/10.1111/j.1474-9726.2012.00832.x; PMID: 22587563
- Livi CB, Hardman RL, Christy BA, Dodds SG, Jones D, Williams C, Strong R, Bokov A, Javors MA, Ikeno Y, et al. Rapamycin extends lifespan of Rb1+/- mice by inhibiting neuroendocrine tumors. Aging (Albany NY) 2013; 5:100 - 10; PMID: 23454836
- Longo VD, Fontana L. Intermittent supplementation with rapamycin as a dietary restriction mimetic. Aging (Albany NY) 2011; 3:1039 - 40; PMID: 22147496
- Zhao C, Vollrath D. mTOR pathway activation in age-related retinal disease. Aging (Albany NY) 2011; 3:346 - 7; PMID: 21483039
- Huang X, Liu J, Withers BR, Samide AJ, Leggas M, Dickson RC. Reducing signs of aging and increasing lifespan by drug synergy. Aging Cell 2013; 12:652 - 60; http://dx.doi.org/10.1111/acel.12090; PMID: 23601176
- Santini E, Valjent E, Fisone G. mTORC1 signaling in Parkinson’s disease and L-DOPA-induced dyskinesia: A sensitized matter. Cell Cycle 2010; 9:2713 - 8; http://dx.doi.org/10.4161/cc.9.14.12180; PMID: 20581466
- Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 2013; 12:851 - 62; http://dx.doi.org/10.1111/acel.12109; PMID: 23734717
- Zhang XM, Li L, Xu JJ, Wang N, Liu WJ, Lin XH, Fu YC, Luo LL. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene 2013; 523:82 - 7; http://dx.doi.org/10.1016/j.gene.2013.03.039; PMID: 23566837
- Glazer HP, Osipov RM, Clements RT, Sellke FW, Bianchi C. Hypercholesterolemia is associated with hyperactive cardiac mTORC1 and mTORC2 signaling. Cell Cycle 2009; 8:1738 - 46; http://dx.doi.org/10.4161/cc.8.11.8619; PMID: 19395857
- Ye L, Widlund AL, Sims CA, Lamming DW, Guan Y, Davis JG, Sabatini DM, Harrison DE, Vang O, Baur JA. Rapamycin doses sufficient to extend lifespan do not compromise muscle mitochondrial content or endurance. Aging (Albany NY) 2013; 5:539 - 50; PMID: 23929887
- Hinojosa CA, Mgbemena V, Van Roekel S, Austad SN, Miller RA, Bose S, Orihuela CJ. Enteric-delivered rapamycin enhances resistance of aged mice to pneumococcal pneumonia through reduced cellular senescence. Exp Gerontol 2012; 47:958 - 65; http://dx.doi.org/10.1016/j.exger.2012.08.013; PMID: 22981852
- Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, Collins FS. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med 2011; 3:89ra58; http://dx.doi.org/10.1126/scitranslmed.3002346; PMID: 21715679
- Wang M, Miller RA. Fibroblasts from long-lived mutant mice exhibit increased autophagy and lower TOR activity after nutrient deprivation or oxidative stress. Aging Cell 2012; 11:668 - 74; http://dx.doi.org/10.1111/j.1474-9726.2012.00833.x; PMID: 22577861
- Kolosova NG, Muraleva NA, Zhdankina AA, Stefanova NA, Fursova AZ, Blagosklonny MV. Prevention of age-related macular degeneration-like retinopathy by rapamycin in rats. Am J Pathol 2012; 181:472 - 7; http://dx.doi.org/10.1016/j.ajpath.2012.04.018; PMID: 22683466
- Gems D, de la Guardia Y. Alternative Perspectives on Aging in Caenorhabditis elegans: Reactive Oxygen Species or Hyperfunction?. Antioxid Redox Signal 2013; 19:321 - 9; http://dx.doi.org/10.1089/ars.2012.4840; PMID: 22870907
- Gems D, Partridge L. Genetics of longevity in model organisms: debates and paradigm shifts. Annu Rev Physiol 2013; 75:621 - 44; http://dx.doi.org/10.1146/annurev-physiol-030212-183712; PMID: 23190075
- Moskalev AA, Shaposhnikov MV. Pharmacological inhibition of phosphoinositide 3 and TOR kinases improves survival of Drosophila melanogaster. Rejuvenation Res 2010; 13:246 - 7; http://dx.doi.org/10.1089/rej.2009.0903; PMID: 20017609
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of lifespan extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab 2010; 11:35 - 46; http://dx.doi.org/10.1016/j.cmet.2009.11.010; PMID: 20074526
- Garelick MG, Mackay VL, Yanagida A, Academia EC, Schreiber KH, Ladiges WC, Kennedy BK. Chronic rapamycin treatment or lack of S6K1 does not reduce ribosome activity in vivo. Cell Cycle 2013; 12:2493 - 504; http://dx.doi.org/10.4161/cc.25512; PMID: 23839034
- Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, et al. Rapamycin Extends Life and Health in C57BL/6 Mice. J Gerontol A Biol Sci Med Sci 2013; http://dx.doi.org/10.1093/gerona/glt056; PMID: 23682161
- Fang Y, Bartke A. Prolonged rapamycin treatment led to beneficial metabolic switch. Aging (Albany NY) 2013; 5:328 - 9; PMID: 23645164
- Passtoors WM, Beekman M, Deelen J, van der Breggen R, Maier AB, Guigas B, Derhovanessian E, van Heemst D, de Craen AJ, Gunn DA, et al. Gene expression analysis of mTOR pathway: association with human longevity. Aging Cell 2013; 12:24 - 31; http://dx.doi.org/10.1111/acel.12015; PMID: 23061800
- Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, Gutkind JS. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell 2012; 11:401 - 14; http://dx.doi.org/10.1016/j.stem.2012.06.007; PMID: 22958932
- Iglesias-Bartolome R, Gutkind SJ. Exploiting the mTOR paradox for disease prevention. Oncotarget 2012; 3:1061 - 3; PMID: 23165441
- Zhou J, Freeman TA, Ahmad F, Shang X, Mangano E, Gao E, Farber J, Wang Y, Ma XL, Woodgett J, et al. GSK-3α is a central regulator of age-related pathologies in mice. J Clin Invest 2013; 123:1821 - 32; http://dx.doi.org/10.1172/JCI64398; PMID: 23549082
- Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, Blagosklonny MV, Gudkov AV. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/- mice. Aging (Albany NY) 2012; 4:709 - 14; PMID: 23123616
- Comas M, Toshkov I, Kuropatwinski KK, Chernova OB, Polinsky A, Blagosklonny MV, Gudkov AV, Antoch MP. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53-/- mice by delaying carcinogenesis. Aging (Albany NY) 2012; 4:715 - 22; PMID: 23117593
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 2013; 497:211 - 6; http://dx.doi.org/10.1038/nature12143; PMID: 23636330
- Tang Y, Cai D. Hypothalamic inflammation and GnRH in aging development. Cell Cycle 2013; 12:2711 - 2; http://dx.doi.org/10.4161/cc.26054; PMID: 23966154
- Perkey E, Fingar D, Miller RA, Garcia GG. Increased Mammalian target of rapamycin complex 2 signaling promotes age-related decline in CD4 T cell signaling and function. J Immunol 2013; 191:4648 - 55; http://dx.doi.org/10.4049/jimmunol.1300750; PMID: 24078700
- Wu X, Cao Y, Nie J, Liu H, Lu S, Hu X, Zhu J, Zhao X, Chen J, Chen X, et al. Genetic and pharmacological inhibition of Rheb1-mTORC1 signaling exerts cardioprotection against adverse cardiac remodeling in mice. Am J Pathol 2013; 182:2005 - 14; http://dx.doi.org/10.1016/j.ajpath.2013.02.012; PMID: 23567640
- Zhang Y, Xu X, Ren J. MTOR overactivation and interrupted autophagy flux in obese hearts: a dicey assembly?. Autophagy 2013; 9:939 - 41; http://dx.doi.org/10.4161/auto.24398; PMID: 23529215
- Rao RR, Li Q, Shrikant PA. Fine-tuning CD8(+) T cell functional responses: mTOR acts as a rheostat for regulating CD8(+) T cell proliferation, survival and differentiation?. Cell Cycle 2010; 9:2996 - 3001; http://dx.doi.org/10.4161/cc.9.15.12359; PMID: 20699660
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature 2009; 460:108 - 12; http://dx.doi.org/10.1038/nature08155; PMID: 19543266
- Nicoletti F, Lapenta C, Donati S, Spada M, Ranazzi A, Cacopardo B, Mangano K, Belardelli F, Perno C, Aquaro S. Inhibition of human immunodeficiency virus (HIV-1) infection in human peripheral blood leucocytes-SCID reconstituted mice by rapamycin. Clin Exp Immunol 2009; 155:28 - 34; http://dx.doi.org/10.1111/j.1365-2249.2008.03780.x; PMID: 19076826
- Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP, Ford ML. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol 2010; 185:2004 - 8; http://dx.doi.org/10.4049/jimmunol.1001176; PMID: 20631309
- Ferrer IR, Araki K, Ford ML. Paradoxical aspects of rapamycin immunobiology in transplantation. Am J Transplant 2011; 11:654 - 9; http://dx.doi.org/10.1111/j.1600-6143.2011.03473.x; PMID: 21446969
- Blagosklonny MV. How to save Medicare: the anti-aging remedy. Aging (Albany NY) 2012; 4:547 - 52; PMID: 22915707
- Blagosklonny MV. Validation of anti-aging drugs by treating age-related diseases. Aging (Albany NY) 2009; 1:281 - 8; PMID: 20157517
- Turner AP, Shaffer VO, Araki K, Martens C, Turner PL, Gangappa S, Ford ML, Ahmed R, Kirk AD, Larsen CP. Sirolimus enhances the magnitude and quality of viral-specific CD8+ T-cell responses to vaccinia virus vaccination in rhesus macaques. Am J Transplant 2011; 11:613 - 8; http://dx.doi.org/10.1111/j.1600-6143.2010.03407.x; PMID: 21342450
- Hill JA, Hummel M, Starling RC, Kobashigawa JA, Perrone SV, Arizón JM, Simonsen S, Abeywickrama KH, Bara C. A lower incidence of cytomegalovirus infection in de novo heart transplant recipients randomized to everolimus. Transplantation 2007; 84:1436 - 42; http://dx.doi.org/10.1097/01.tp.0000290686.68910.bd; PMID: 18091519
- Kobashigawa J, Ross H, Bara C, Delgado JF, Dengler T, Lehmkuhl HB, Wang SS, Dong G, Witte S, Junge G, et al. Everolimus is associated with a reduced incidence of cytomegalovirus infection following de novo cardiac transplantation. Transpl Infect Dis 2013; 15:150 - 62; http://dx.doi.org/10.1111/tid.12007; PMID: 23013440
- Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer 2011; 104:643 - 52; http://dx.doi.org/10.1038/bjc.2011.15; PMID: 21285988