The CD34 antigen has long been used to define human hematopoietic stem and progenitor cells (HSPCs), however, a hematopoietic cell population that is negative for CD34 expression has also been shown to be able to repopulate immunodeficient mice.Citation1 Our laboratory has previously demonstrated that only the CD93hi (mouse homolog of AA4.1 or C1qRp) sub-fraction within the Lin−CD34−CD38− cell population has repopulating capacity in immunodeficient mice.Citation2 Despite the identification of this population, little was known about the molecular characteristics of these rare CD34− cells or the relationship to the CD34+ populations. The self-renewal features of these Lin−CD34−CD38−CD93+ (−/−/+) cells have been demonstrated only recently, including the characterization of some of their cellular and molecular features.Citation3 Different than any of Lin−CD34+CD38− (+/−) sub-fractions,Citation3-Citation5 when uncultured, −/−/+ cells have almost no clonogenic capacity and produce a delayed and low level of primary engraftment at 12 wk post-transplant. Yet, they are more competent long-term and in serial transplant than +/− cells in vivo.Citation3 These and other features demonstrate that the −/−/+ population contains bona fide HSCs and can be placed in the human hematopoietic hierarchy above the commonly known +/− HSPCs, at least from umbilical cord blood (CB).
The population of −/−/+ cells are highly quiescent as they express high levels of important cell cycle regulators that maintain the G0 state and block the G0–G1 progression, in particular E2F4, RBL2 (p130), and CDKN1C (p57KIP2). Furthermore, the expression of important HSC developmental regulators such as LMO2, GATA2, RUNX1A, and TALl are low, suggesting that the −/−/+ population represents an immature cell type. The Notch and TGFβ pathways, at least in part, regulate these unique features. The −/−/+ cells demonstrate a unique Notch4 expression and cleavage pattern. When these cells interact with Delta-like 4, but not with Delta-like 1 or Jagged1 ligands, the upregulation of CD34 expression and induction of clonogenic activity are halted, which is accompanied by the repression of developmental regulators ().
Figure 1. Graphic representation of the signaling pathways involved in the regulation of −/−/+ HSCs. Hypothetical Notch ligand-receptor pairing (?) involved in the Notch signaling in −/−/+ HSCs.
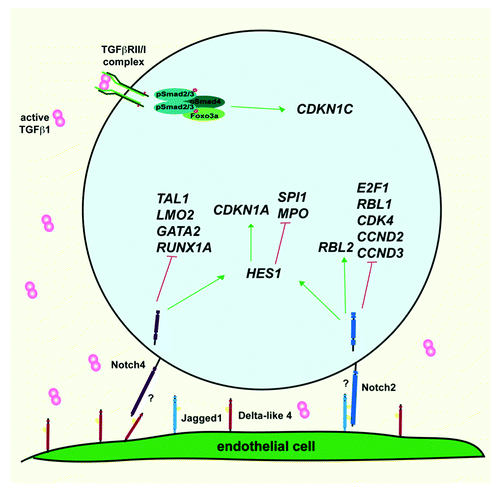
With respect to cell cycle, a combination of Jagged1 and TGF-β signaling plays a role in regulating the quiescent state of −/−/+ cells. Notch signaling driven by Jagged1 binding can sustain these cells in G0 by blocking the G0–G1 transition (repressing E2F1, RBL1 [p107], CDK4, CDK6, CCND2, and CCND3 expression), while TGFβ1 stimulation can sustain p57KIP2 expression (). As considerable TGFβ signaling was found in −/−/+ cells, we believe that p57KIP2 might be one of the key factors that prevent −/−/+ cells from entering and progressing through G1 phase.Citation3 More importantly, −/−/+ cells are sensitive to pharmacological inhibition of Notch and TGFβ in vivo, demonstrating that these pathways play a role in controlling the differentiation and cell cycle progression of −/−/+ HSCs in vivo. One of the most interesting findings from our recent work is that we observed that this rare stem cell population has these pathways active, remaining undifferentiated in vivo in the mouse bone marrow (BM) for up to 6 wk after transplantation. However, the stimuli that triggers them to “start kicking” in vivo is still unclear. If we can unravel this trigger, it may lead to a better understanding of the signals that control the switches between quiescence and cycling, and self-renewal and differentiation, during homeostasis. Such an understanding can also provide important clues on how these cells differentiate into functional CD34+ cells in vivo, allowing their use in cell expansion and production of +/− HSCs in vitro. Indeed, it is tempting to speculate that these CD34− stem cells should be a better source of HSCs for expansion, as they can self-renew and also give rise by differentiation to bone fide CD34+ HSCs, capable of multi-lineage engraftment in the xenotransplantation model. Nevertheless more studies will be needed to reveal factors able to push these cells into cycle. Analysis of these cells during ontogeny might also give us some clues. It has been well documented that during early HSC development, the HSC compartment is highly cycling. Are these CD34− stem cells present in embryonic and fetal hematopoietic tissues? And if so, are these cells cycling? Answering these key questions should shed light into ways to better expand bone fide human HSCs.
In summary, although further investigation is needed to provide greater evidence of the specific role of −/−/+ HSCs in human adult hematopoiesis, their existence in adult bone marrow (with features comparable to their CB counterparts)Citation1,Citation3 leads us to propose that these −/−/+ HSCs could represent the last reservoir of HSCs, where their contribution might only be required during conditions of stress rather than steady-state hematopoiesis.Citation6
References
- Bhatia M, et al. Nat Med 1998; 4:1038 - 45; http://dx.doi.org/10.1038/2023; PMID: 9734397
- Danet GH, et al. Proc Natl Acad Sci U S A 2002; 99:10441 - 5; http://dx.doi.org/10.1073/pnas.162104799; PMID: 12140365
- Anjos-Afonso F, et al. Cell Stem Cell 2013; 13:161 - 74; http://dx.doi.org/10.1016/j.stem.2013.05.025; PMID: 23910083
- Notta F, et al. Science 2011; 333:218 - 21; http://dx.doi.org/10.1126/science.1201219; PMID: 21737740
- Doulatov S, et al. Cell Stem Cell 2012; 10:120 - 36; http://dx.doi.org/10.1016/j.stem.2012.01.006; PMID: 22305562
- Copley MR, et al. Cell Stem Cell 2012; 10:690 - 7; http://dx.doi.org/10.1016/j.stem.2012.05.006; PMID: 22704509
