Squamous cell carcinomas (SCCs) are the most frequent type of solid cancers affecting a broad range of tissues, such as skin, lung, oral cavity and genital tract, and, less frequently, bladder and thyroid. Their common feature is that they arise from stratified or pseudo-stratified epithelia and are highly heterogeneous and often associated with a high degree of differentiation, making them resilient to cancer therapy. One organ of choice to decipher the squamous differentiation program is the skin. In the basal layer of the epidermis, a dynamic equilibrium exists among populations with high self-renewal potential and cells at different stages of commitment to differentiation. This equilibrium is essential for long-term tissue homeostasis and prevention of carcinogenesis. Importantly, we previously showed that p53 activation in keratinocytes of the proliferative compartment triggers a pro-differentiation program, while its disruption accounts for the deranged differentiation program observed in keratinocyte-derived cancer cells. Notably, besides p53, only a few other driver genes are mutated in SCCs, pointing to the importance of epigenetics in this cancer type.
microRNAs (miRNAs) provide an important form of epigenetic control of gene expression frequently deranged in cancer. miRNAs are 17–25 nucleotide non-coding RNA molecules that mostly bind to the 3′ untranslated regions (UTR) of target mRNAs in a sequence specific manner in order to influence translation and/or transcript stability. They are often expressed in a lineage- and time-specific fashion and have the potential to control cell fate decisions.
In our recent work aimed at identifying miRNAs deregulated in cutaneous SCCs, we showed that levels of 2 specific miRNAs, miR-34a and miR-203, are consistently reduced.Citation1 While miR-203 has been intensively studied as a key inducer of keratinocyte differentiation limiting stemness,Citation2 the role of miR-34a in this process has been poorly explored. miR-34a is better known as a mediator of p53 action on growth arrest, senescence, apoptosis, and epithelial–mesenchymal transition.Citation3 miR-34a maps to the 1p36 genomic region that is frequently deleted in human cancers, and its expression is downmodulated in a variety of cancers.
We found that miR-34a expression is induced with keratinocyte differentiation, and that its reduced expression in cutaneous SCCs can be explained by p53 loss of function as well as methylation of its promoter. miR-34a mediates the p53 pro-differentiation effects in keratinocytes. Its increased expression, to levels similar to those found in differentiating keratinocytes, is sufficient to induce important aspects of the differentiation program through a mechanism that can be uncoupled from induction of cell cycle arrest.
In search of miR-34a direct targets mediating its pro-differentiation function, we interrogated several genes downregulated by miR-34a and harboring a miR-34a binding sequence in their 3′UTR. Notch1, a transmembrane receptor known to have a positive role on keratinocyte differentiation, fulfilled these criteria, and its repression by miR-34a could not account for the pro-differentiation function of miR-34a. Instead, miR-34a participates in the fine-tuned regulation of Notch1 expression by p53. Another previously reported target of miR-34a is SIRT1, and an attractive hypothesis was that Sirtuins could mediate the miR-34a pro-differentiation function. Sirtuins are protein deacetylases and/or ADP-ribosyl transferases involved in a broad range of biological processes, like development/differentiation, chromatin remodeling, metabolism, DNA repair and cell survival.Citation4 SIRT1, a known miR-34a target in other cells, was only slightly regulated by miR-34a overoverexpression in keratinocytes. By contrast, SIRT6, the only other sirtuin family member that we found to have a miR-34a putative binding site in its 3′-UTR region, was downregulated by increased amounts of miR-34a. Silencing SIRT6 in primary keratinocytes and SCC cells could recapitulate at least partially the effects of miR-34a on differentiation, and SIRT6 overexpression counteracted the miR-34a pro-differentiation effects, making it a likely player in keratinocyte differentiation (). These findings are of possible clinical relevance, as opposed to miR-34a, we found that SIRT6 levels decrease during keratinocyte differentiation, while SIRT6 is overexpressed in human precancerous lesions (actinic keratoses) as well as more advanced SCCsCitation1
Figure 1. miR-34a mediates p53 pro-differentiation effects with subsequent SIRT6 silencing in keratinocytes. Top: In normal epidermis, p53 activation promotes differentiation in cells of the proliferative compartment through increased expression of miR-34a, which inhibits SIRT6 expression. In SCCs, loss of p53 and miR-34a result in SIRT6 protein accumulation and impaired differentiation. Bottom: Mechanistically, upon differentiating signals, p53 activates transcriptionally miR-34a expression, which, in turn, inhibits SIRT6 expression via direct binding to its 3′UTR.
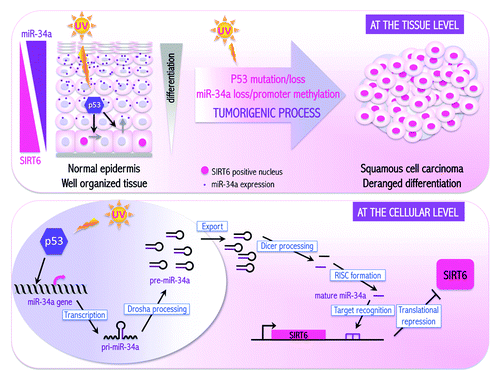
SIRT6 plays a role in DNA repair, genomic stability, and glucose metabolismCitation4 and has been described as a tumor suppressor gene in hepatocellular and colorectal cancers.Citation5,Citation6 Our findings suggest that SIRT6 could play an opposite and/or more complex function in keratinocytes that needs further investigations. For instance, whether overexpression of SIRT6 promotes keratinocyte tumor development has not yet been explored. Also, what are the mechanisms involved in the regulation of differentiation by SIRT6? Which functions of SIRT6 are required? While a possible tumor modulatory function of SIRT6 in skin and other tissues needs to be further investigated, a few recent reports have indicated that this protein is more expressed in tumor cell lines than corresponding normal cells. Notably, the negative correlation between SIRT6 and miR-34a expression that we have uncovered might apply to aging as well as to somatic cell reprogramming, which is restrained by miR-34a while enhanced by SIRT6.Citation7,Citation8 Given the targetable property of Sirtuins, the development of SIRT6-specific inhibitors might be of therapeutic value for treatment of premalignant and malignant SCCs and beyond.
Acknowledgements
This work was supported by by grants from the Swiss National Science Foundation (310030B/138653/1), NIH (AR39190), and Oncosuisse (OCS-2922-02-2012).
References
- Lefort K, et al. EMBO J 2013; 32:2248 - 63; http://dx.doi.org/10.1038/emboj.2013.156; PMID: 23860128
- Yi R, et al. Nature 2008; 452:225 - 9; http://dx.doi.org/10.1038/nature06642; PMID: 18311128
- Hermeking H. Nat Rev Cancer 2012; 12:613 - 26; http://dx.doi.org/10.1038/nrc3318; PMID: 22898542
- Martinez-Pastor B, et al. Front Pharmacol 2012; 3:22; http://dx.doi.org/10.3389/fphar.2012.00022; PMID: 22363287
- Min L, et al. Nat Cell Biol 2012; 14:1203 - 11; http://dx.doi.org/10.1038/ncb2590; PMID: 23041974
- Sebastián C, et al. Cell 2012; 151:1185 - 99; http://dx.doi.org/10.1016/j.cell.2012.10.047; PMID: 23217706
- Choi YJ, et al. Nat Cell Biol 2011; 13:1353 - 60; http://dx.doi.org/10.1038/ncb2366; PMID: 22020437
- Sharma A, et al. J Biol Chem 2013; 288:18439 - 47; http://dx.doi.org/10.1074/jbc.M112.405928; PMID: 23653361
