Abstract
The conventional view of CD95 (Fas/APO-1) is that it is a dedicated apoptosis-inducing receptor with important functions in immune cell homeostasis and in viral and tumor defense. There is an emerging recognition, however, that CD95 also has multiple non-apoptotic activities. In the context of cancer, CD95 was shown to have tumor-promoting activities, and the concept of this new function of CD95 in cancer is gaining traction. Recently, we showed that not only is CD95 a growth promoter for cancer cells, but, paradoxically, when either CD95 or CD95 ligand (CD95L) is removed, that virtually all cancer cells die through a process we have named DICE (death induced by CD95R/L elimination). In this perspective, I outline a hypothesis regarding the physiological function of DICE, and why it may be possible to use induction of DICE to treat many, if not most, cancers.
Keywords: :
The Proapoptotic Function of CD95 and CD95L in Normal Cells
CD95 is an established apoptosis-inducing death receptor, and the details of the signaling pathway mediating apoptosis are well studied.Citation1,Citation2 In immune cells, the CD95/CD95L system is involved primarily in the downregulation of immune responses.Citation3 This was established through the analysis of 3 mutant mouse strains. Mice with lymphoproliferative disease (lpr mice) express only about 10% of the level of normal CD95 due to a retroviral insertion into the gene. These animals are phenotypically similar to mice with generalized lymphoproliferative disorder (gld), which express a nonfunctional CD95L, and to mice carrying a point mutation in the CD95 death domain (lprcg mice). All of these mice exhibit accumulation of nondeleted lymphocytes and suffer from autoimmune manifestations (reviewed in ref. Citation3). In humans, a similar syndrome has been described.Citation4-Citation8 Autoimmune lymphoproliferative syndrome (ALPS) type Ia patients carry heterozygous dominant-negative mutations in CD95; ALPS type Ib patients have mutations in CD95L, and ALPS type II patients have mutations in caspase-10. The most well-established pro-apoptotic activity of CD95 is to instruct virus-infected cells or cancer cells to die via apoptosis when engaged by cytotoxic killer cells or other immune cells that use CD95L.Citation9 Thus, the fact that most cancer cells express CD95, sometimes in large quantities,Citation10 is something of a surprise.
Nonapoptotic Signaling Through CD95 in Normal Cells and in Cancer
There is growing evidence that CD95 plays an apoptosis-independent role in nonimmune cells and in solid cancers.Citation11-Citation13 In fact, CD95 acts in some tissues as a survival factor. For example, CD95 is required for efficient liver regeneration following partial hepatectomy;Citation14,Citation15 CD95 is important for neurite outgrowth,Citation16,Citation17 and CD95 is a potent activator of neurogenesis in both healthy and injured brains, where it activates neuronal stem cells.Citation18 The expression of CD95 on cancer cells suggests that cancer cells maintain CD95 expression for activities other than apoptosis induction. Consistent with this assumption are data demonstrating roles for CD95 in promoting cancer cell migration, growth, epithelial-to-mesenchymal transition (EMT), and development, as shown in lung cancer,Citation19,Citation20 colon cancer,Citation21-Citation24 gastrointestinal cancers,Citation25,Citation26 pancreatic cancer,Citation27,Citation28 glioblastoma multiforme (GBM),Citation29 and in 22 cell lines representing various cancers.Citation30 Our most recent finding is that cancer fails to develop in mouse models of low-grade or endometrioid ovarian cancer or liver cancer unless CD95 is expressed,Citation14,Citation31 suggesting that the reason cancer cells usually express CD95 goes beyond a function of CD95 as a tumor promoter.
The Role of CD95L in Elimination Cancer Cells
Apoptosis induced through CD95L is one of the mechanisms used by the immune system to kill virus infected and cancerous cells.Citation32 The role of CD95L used by CD8+ T cells to control viral infections is well established. As one example, adoptive transfer of West Nile virus primed CD8+ T cells from wild-type but not from CD95L-deficient gld mice efficiently limited infection of the virus in the CNS and enhanced survival rates.Citation33 However, the putative role of CD95L expressing CD8+ T cells to eliminate cancer cells is more controversial.
In the early 1980s it was recognized that CD8-positive cytotoxic lymphocytes (CTL) kill cancer cells in an antigen- and Ca2+-dependent fashion.Citation34 The pathway that was identified as mediating Ca2+-dependent cytolytic activity was the granule pathway involving the transfer of granzymes into target cells with the help of perforin (Pfp). Indeed, T cells and NK cells from perforin knockout mice were later shown to have impaired ability to kill target cells.Citation35 Even earlier, a Ca2+-independent cytolytic pathway was postulated.Citation36 Shortly after CD95 was identified as a major apoptosis-inducing receptor engaged by immune cells, it was reported that CD95 on target cells and CD95L in CTLs was responsible for the long sought-after Ca2+-independent mechanism of T cell-mediated cytotoxicity.Citation37 It was shown in a number of systems that these 2 pathways, the Pfp/granzyme pathway and the CD95/CD95L ligand pathway, could account for most cytolytic activity seen in CTLs.Citation38-Citation40 However, most of these early studies only tested the activity of CTLs in vitro using effector target kill assays. These in vitro findings were generally confirmed, but it was recognized that the situation in vivo might be more complicated. Tumor regression of Renca cells grown in syngeneic BALB/c mice was independent of Pfp, but was enhanced by both interferon (IFN)γ and TNFα.Citation41 CD95 was found to be critical for mediating tumor regression in a number of models of liver cancerCitation42 and models of experimental lung metastasis.Citation43,Citation44 Upregulation of CD95 induced by irradiation of tumor cells was shown to enhance cytolytic activities of CTLs during adoptive immunotherapy.Citation45
In addition to the Pfp/granzyme and the CD95/CD95L systems, evidence of a third cytolytic activity was found, as CTLs deficient in both functional CD95L and Pfp showed residual cytolytic activity.Citation46 This finding was supported by other studies suggesting that CTL killing of tumor cells was possible without either of the 2 main systems. T cell-mediated tumor regression and long-term antitumor immunity in mouse models of melanoma and sarcoma were found to be independent of Pfp and CD95L. This was established using adoptive transfer of effector T cells from either perforin-deficient or from gld mice.Citation47 In a lung cancer mouse model, Lewis lung cancer cells were rejected in vivo in a manner dependent on CD8+ T cells that killed cancer cells independently of perforin or CD95L.Citation48 Depending on the tumor models studied, antitumor activity was found to depend on IFNγ,Citation49,Citation50 IFNγ and CD95L,Citation51 first IFNγ then Pfp in a 2-hit model,Citation52 IFNγ and TNFα,Citation53 or TNFα.Citation54 One study suggested that neither Pfp nor CD95 nor TNFα were involved.Citation55 In addition to the role of CD95 as one of a number of mechanisms employed by cytolytic CD8+ T cells, CD95 has been reported to be the primary mechanism utilized by many CD4+ lytic effectors.Citation56,Citation57 Most recently, Blankenstein and colleagues have revisited the role of CD95 in the elimination of established larger tumors using adoptive transfer of tumor specific T cells.Citation58 Often during immune therapy, after an initial tumor regression, the cancer relapses due to the outgrowth of antigen-loss variants (ALVs). Interestingly, in this study not only had the ALVs reduced CD95 expression, supporting a role of CD95 in the elimination of the cancer cells by CTLs, but the cells of the tumor stroma were also found to have downregulated CD95. While further analysis will be required to determine the role of CD95 in the elimination of cancer cells, there is strong evidence that the CD95/CD95L system is an important part of the antitumor defense.
Using CD95 for Cancer Therapy
The discovery in the late 1980s of 2 antibodies that could kill cancer cellsCitation59,Citation60 was the beginning of a new era. For the first time, it became feasible to kill cancer cells by triggering an endogenous protein expressed on most cancer cells. The initial excitement was high regarding the discovery of a natural pathway the body could use to kill neoplastically transformed cells. CD95 was confirmed to be one of the major death receptors used by immune cells to kill cancer cells. However, it soon became clear that apoptosis could not be selectively induced in cancer cells without affecting normal tissues such as the liver.Citation61 Equally disheartening, most cancer cells are resistant to CD95-mediated apoptosis.Citation10
Enter DICE
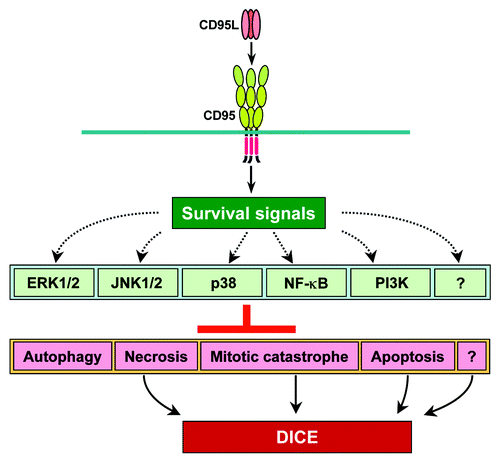
When either CD95 or CD95L were downregulated by siRNAs or shRNAs, growth of all tested tumors cells slowed down,Citation14 and if the knockdown was sustained for a sufficient length of time, the tumor cells died.Citation31 We reported that a substantial fraction of all tested cancer cells underwent death induced by CD95/CD95L elimination (DICE).Citation31 DICE is characterized by swelling of the cells, the appearance of large dilated vacuoles, signs of autophagy, generation of ROS, and DNA DSBs. This is followed by a reduction in cell viability, activation of caspase-2, mitochondrial outer membrane permeabilization (MOMP), and, ultimately, cell death.Citation31 DICE resembles a necrotic form of mitotic catastrophe. Interestingly, by performing a small-molecule screen and a genome-wide shRNA screen we did not find a single drug or a single gene that would inhibit or promote DICE.Citation31 It is interesting that none of the multiple nonapoptotic signaling pathways that have been found to be engaged by CD95 were found, either alone or in combination, to be responsible for keeping cancer cells alive. Based on these data, I hypothesize that CD95 acts as a lynchpin to allow various cell survival pathways to signal at a low level (). The moment the connection between CD95 and any (or all) of these pathways is disrupted cancer cells will turn on the DICE program and die. DICE represents multiple cell death pathways, culminating in mitotic catastrophe, and may also involve induction of senescence based on changes in morphology seen in all cancer cells undergoing DICE.Citation31 This would also be consistent with a reported mTOR-regulated connection between mitotic catastrophe and cellular senescence.Citation62 This, however, requires further testing. Based on data from both CD95 and CD95L knockout mice, which do not show signs of cell death or growth deficiencies in any tissue outside the immune system,Citation63-Citation65 DICE may preferentially affect cancer cells and have little effect on normal cells. This prediction is supported by the finding that hTERT immortalized fibroblasts were more susceptible than nonimmortalized fibroblasts to DICE induced by either CD95 or CD95L knockdown.Citation31 This suggests that dependence on CD95 and CD95L for survival is a feature of cancer cells that distinguishes them from most normal cells. The few normal cells that seem to be affected in their growth/viability after elimination of CD95 are proliferating T cellsCitation66 and hepatocytes in the regenerating liver,Citation14 both tissues prone to neoplastic transformation.
The Physiological Function of DICE, a Hypothesis
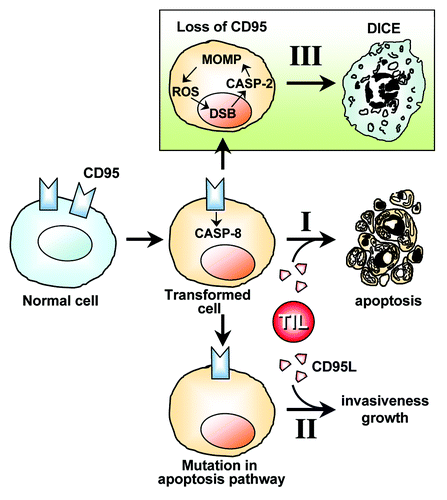
As summarized above, it appears that cells of the immune system employ well-established activities of CD95/CD95L in the elimination of neoplastically transformed cells. Infiltrating immune cells kill cancer cells by activating the canonical caspase-8-dependent apoptosis pathway in the cancer cells (, I). On rare occasions, this process fails due to mutations or epigenetic deregulation of genes in the cancer cells, rendering them resistant to CD95 mediated apoptosis. Accumulating data suggest that under these conditions, stimulation of CD95 on the tumor cells by CD95L drives tumor growth, motility, and dedifferentiation of the cancer cells (, II). When a deletion or mutation of CD95 occurs in tumor cells or rapidly proliferating cells (i.e., T cells or regenerating liver cells), DICE is turned on (, III). DICE is, therefore, a fail-safe mechanism that prevents the survival of cells that are devoid of CD95 and, hence, cannot be eliminated by the immune system through CD95L/CD95-mediated apoptosis. The observation that tumor cells rarely if ever mutate or delete both alleles of CD95 (reviewed in ref. Citation67) is consistent with this interpretation.
Figure 2. Hypothesis on the physiological relevance of DICE. CD95L-induced apoptosis is one of the mechanisms that tumor-infiltrating lymphocytes (TILs) employ to eliminate transformed cells (I). If CD95 engagement fails to induce apoptosis due to cancer cells acquiring mutations of components of the apoptosis pathways, then cancer can develop. These cancer cells now benefit from the CD95L expressed by themselves and, presumably, by TILs (II). Transformed cells and cells in rapidly proliferating tissues under conditions that can lead to neoplastic transformation (e.g., compensatory proliferation of liver tissue) must receive a low level signal through CD95 to survive. If CD95 or its stimulating ligand are completely removed, then cells die by DICE (III). According to this model, CD95 and CD95L are part of a fail-safe mechanism to prevent the occurrence of CD95-deficient cancer cells that can no longer be targeted by immune cells via CD95L. TIL, tumor-infiltrating lymphocyte secreting CD95L. DSB, DNA double-strand breaks.
DICE as Cancer Therapeutics
Developing new cancer therapeutics is often based on the following strategy: A gene or a gene product (RNA or protein) is found to be amplified or upregulated or mutated in primary tumors, metastases, or recurrent cancer; or a gene is identified as a so-called driver mutation by genome-wide analyses. The suspect gene is studied functionally to determine whether it has tumorigenic activities, and then is tested in mouse tumor models and analyzed using patient material for its usefulness as a prognostic marker. If it passes these tests, it is viewed as an oncogene and considered as a potential biomarker or a target for cancer therapy. Despite the wide acceptance of this approach, the fact remains that targeting oncogenes clinically has only been partially successful and only in a few cancers (e.g., BCR-ABL by Imatinib in CMLCitation68 or B-RAF[V600E] by Vemurafenib in melanoma).Citation69 The induction of DICE represents a different concept for cancer therapy. To induce DICE, I propose targeting CD95L and/or CD95, which are widely considered to have tumor-suppressive activities and are often downregulated (albeit only to a basal level) in cancer. Based on our data, I further hypothesize that tumor cells will not readily develop resistance to DICE. DICE is not a mechanism that prevents tumor development; thus, any tumor that develops has not been under selection pressure from DICE. DICE only prevents survival of existing tumor cells that lose CD95 functionality and, therefore, cannot be eliminated by immune cells. Inducing DICE in tumor cells by targeting CD95 or CD95L could be a novel and powerful means to eradicate cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 2003; 10:26 - 35; http://dx.doi.org/10.1038/sj.cdd.4401186; PMID: 12655293
- Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet 1999; 33:29 - 55; http://dx.doi.org/10.1146/annurev.genet.33.1.29; PMID: 10690403
- Peter ME, Barnhart BC, Algeciras-Schimnich A. The Cytokine Handbook: CD95L/FasL and its receptor CD95 (APO-1/Fas). The Cytokine Handbook 2003; 2:885 - 911; http://dx.doi.org/10.1016/B978-012689663-3/50042-9
- Rieux-Laucat F, Blachère S, Danielan S, De Villartay JP, Oleastro M, Solary E, Bader-Meunier B, Arkwright P, Pondaré C, Bernaudin F, et al. Lymphoproliferative syndrome with autoimmunity: A possible genetic basis for dominant expression of the clinical manifestations. Blood 1999; 94:2575 - 82; PMID: 10515860
- Drappa J, Vaishnaw AK, Sullivan KE, Chu JL, Elkon KB. Fas gene mutations in the Canale-Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N Engl J Med 1996; 335:1643 - 9; http://dx.doi.org/10.1056/NEJM199611283352204; PMID: 8929361
- Martin DA, Zheng L, Siegel RM, Huang B, Fisher GH, Wang J, Jackson CE, Puck JM, Dale J, Straus SE, et al. Defective CD95/APO-1/Fas signal complex formation in the human autoimmune lymphoproliferative syndrome, type Ia. Proc Natl Acad Sci U S A 1999; 96:4552 - 7; http://dx.doi.org/10.1073/pnas.96.8.4552; PMID: 10200300
- Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 1995; 268:1347 - 9; http://dx.doi.org/10.1126/science.7539157; PMID: 7539157
- Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rösen-Wolff A, Peters AM, Sneller MC, Hallahan CW, Wang J, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood 2001; 98:194 - 200; http://dx.doi.org/10.1182/blood.V98.1.194; PMID: 11418480
- Berke G. Killing mechanisms of cytotoxic lymphocytes. Curr Opin Hematol 1997; 4:32 - 40; http://dx.doi.org/10.1097/00062752-199704010-00006; PMID: 9050377
- Algeciras-Schimnich A, Pietras EM, Barnhart BC, Legembre P, Vijayan S, Holbeck SL, Peter ME. Two CD95 tumor classes with different sensitivities to antitumor drugs. Proc Natl Acad Sci U S A 2003; 100:11445 - 50; http://dx.doi.org/10.1073/pnas.2034995100; PMID: 14504390
- Peter ME, Budd RC, Desbarats J, Hedrick SM, Hueber AO, Newell MK, Owen LB, Pope RM, Tschopp J, Wajant H, et al. The CD95 receptor: apoptosis revisited. Cell 2007; 129:447 - 50; http://dx.doi.org/10.1016/j.cell.2007.04.031; PMID: 17482535
- Wajant H, Pfizenmaier K, Scheurich P. Non-apoptotic Fas signaling. Cytokine Growth Factor Rev 2003; 14:53 - 66; http://dx.doi.org/10.1016/S1359-6101(02)00072-2; PMID: 12485619
- Martin-Villalba A, Llorens-Bobadilla E, Wollny D. CD95 in cancer: tool or target?. Trends Mol Med 2013; 19:329 - 35; http://dx.doi.org/10.1016/j.molmed.2013.03.002; PMID: 23540716
- Chen L, Park SM, Tumanov AV, Hau A, Sawada K, Feig C, Turner JR, Fu YX, Romero IL, Lengyel E, et al. CD95 promotes tumour growth. Nature 2010; 465:492 - 6; http://dx.doi.org/10.1038/nature09075; PMID: 20505730
- Desbarats J, Newell MK. Fas engagement accelerates liver regeneration after partial hepatectomy. Nat Med 2000; 6:920 - 3; http://dx.doi.org/10.1038/78688; PMID: 10932231
- Desbarats J, Birge RB, Mimouni-Rongy M, Weinstein DE, Palerme JS, Newell MK. Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat Cell Biol 2003; 5:118 - 25; http://dx.doi.org/10.1038/ncb916; PMID: 12545171
- Zuliani C, Kleber S, Klussmann S, Wenger T, Kenzelmann M, Schreglmann N, Martinez A, del Rio JA, Soriano E, Vodrazka P, et al. Control of neuronal branching by the death receptor CD95 (Fas/Apo-1). Cell Death Differ 2006; 13:31 - 40; http://dx.doi.org/10.1038/sj.cdd.4401720; PMID: 16003386
- Corsini NS, Sancho-Martinez I, Laudenklos S, Glagow D, Kumar S, Letellier E, Koch P, Teodorczyk M, Kleber S, Klussmann S, et al. The death receptor CD95 activates adult neural stem cells for working memory formation and brain repair. Cell Stem Cell 2009; 5:178 - 90; http://dx.doi.org/10.1016/j.stem.2009.05.004; PMID: 19664992
- Lee JK, Sayers TJ, Back TC, Wigginton JM, Wiltrout RH. Lack of FasL-mediated killing leads to in vivo tumor promotion in mouse Lewis lung cancer. Apoptosis 2003; 8:151 - 60; http://dx.doi.org/10.1023/A:1022918625509; PMID: 12766475
- Zhang Y, Liu Q, Zhang M, Yu Y, Liu X, Cao X. Fas signal promotes lung cancer growth by recruiting myeloid-derived suppressor cells via cancer cell-derived PGE2. J Immunol 2009; 182:3801 - 8; http://dx.doi.org/10.4049/jimmunol.0801548; PMID: 19265159
- Hoogwater FJ, Nijkamp MW, Smakman N, Steller EJ, Emmink BL, Westendorp BF, Raats DA, Sprick MR, Schaefer U, Van Houdt WJ, et al. Oncogenic K-Ras turns death receptors into metastasis-promoting receptors in human and mouse colorectal cancer cells. Gastroenterology 2010; 138:2357 - 67; http://dx.doi.org/10.1053/j.gastro.2010.02.046; PMID: 20188103
- Nijkamp MW, Hoogwater FJ, Steller EJ, Westendorp BF, van der Meulen TA, Leenders MW, Borel Rinkes IH, Kranenburg O. CD95 is a key mediator of invasion and accelerated outgrowth of mouse colorectal liver metastases following radiofrequency ablation. J Hepatol 2010; 53:1069 - 77; http://dx.doi.org/10.1016/j.jhep.2010.04.040; PMID: 20832890
- Park SM, Chen L, Zhang M, Ashton-Rickardt P, Turner JR, Peter ME. CD95 is cytoprotective for intestinal epithelial cells in colitis. Inflamm Bowel Dis 2010; 16:1063 - 70; http://dx.doi.org/10.1002/ibd.21195; PMID: 20049944
- Li H, Fan X, Stoicov C, Liu JH, Zubair S, Tsai E, Ste Marie R, Wang TC, Lyle S, Kurt-Jones E, et al. Human and mouse colon cancer utilizes CD95 signaling for local growth and metastatic spread to liver. Gastroenterology 2009; 137:934 - 44, e1-4; http://dx.doi.org/10.1053/j.gastro.2009.06.004; PMID: 19524576
- Zheng H, Li W, Wang Y, Liu Z, Cai Y, Xie T, Shi M, Wang Z, Jiang B. Glycogen synthase kinase-3 beta regulates Snail and β-catenin expression during Fas-induced epithelial-mesenchymal transition in gastrointestinal cancer. Eur J Cancer 2013; 49:2734 - 46; http://dx.doi.org/10.1016/j.ejca.2013.03.014; PMID: 23582741
- Zheng HX, Cai YD, Wang YD, Cui XB, Xie TT, Li WJ, Peng L, Zhang Y, Wang ZQ, Wang J, et al. Fas signaling promotes motility and metastasis through epithelial-mesenchymal transition in gastrointestinal cancer. Oncogene 2013; 32:1183 - 92; http://dx.doi.org/10.1038/onc.2012.126; PMID: 22508480
- Yuan K, Jing G, Chen J, Liu H, Zhang K, Li Y, Wu H, McDonald JM, Chen Y. Calmodulin mediates Fas-induced FADD-independent survival signaling in pancreatic cancer cells via activation of Src-extracellular signal-regulated kinase (ERK). J Biol Chem 2011; 286:24776 - 84; http://dx.doi.org/10.1074/jbc.M110.202804; PMID: 21613217
- Trauzold A, Röder C, Sipos B, Karsten K, Arlt A, Jiang P, Martin-Subero JI, Siegmund D, Müerköster S, Pagerols-Raluy L, et al. CD95 and TRAF2 promote invasiveness of pancreatic cancer cells. FASEB J 2005; 19:620 - 2; PMID: 15670977
- Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O, Thiemann M, Mueller W, Sykora J, Kuhn A, et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell 2008; 13:235 - 48; http://dx.doi.org/10.1016/j.ccr.2008.02.003; PMID: 18328427
- Barnhart BC, Legembre P, Pietras E, Bubici C, Franzoso G, Peter ME. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J 2004; 23:3175 - 85; http://dx.doi.org/10.1038/sj.emboj.7600325; PMID: 15272306
- Hadji A, Ceppi P, Murmann AE, Brockway S, Pattanayak A, Bhinder B, Hau A, De Chant S, Parimi V, Kolesza P, et al. Death induced by CD95 or CD95 ligand elimination. Cell Rep 2014; In press http://dx.doi.org/10.1016/j.celrep.2014.02.035; PMID: 24656822
- Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity 2009; 30:180 - 92; http://dx.doi.org/10.1016/j.immuni.2009.01.001; PMID: 19239902
- Shrestha B, Diamond MS. Fas ligand interactions contribute to CD8+ T-cell-mediated control of West Nile virus infection in the central nervous system. J Virol 2007; 81:11749 - 57; http://dx.doi.org/10.1128/JVI.01136-07; PMID: 17804505
- Tirosh R, Berke G. T-Lymphocyte-mediated cytolysis as an excitatory process of the target. I. Evidence that the target cell may be the site of Ca2+ action. Cell Immunol 1985; 95:113 - 23; http://dx.doi.org/10.1016/0008-8749(85)90300-4; PMID: 3928177
- Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 1994; 369:31 - 7; http://dx.doi.org/10.1038/369031a0; PMID: 8164737
- MacLennan IC, Gotch FM, Golstein P. Limited specific T-cell mediated cytolysis in the absence of extracellular Ca2+. Immunology 1980; 39:109 - 17; PMID: 6769782
- Rouvier E, Luciani MF, Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med 1993; 177:195 - 200; http://dx.doi.org/10.1084/jem.177.1.195; PMID: 7678113
- Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 1994; 370:650 - 2; http://dx.doi.org/10.1038/370650a0; PMID: 7520535
- Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 1994; 265:528 - 30; http://dx.doi.org/10.1126/science.7518614; PMID: 7518614
- Kojima H, Shinohara N, Hanaoka S, Someya-Shirota Y, Takagaki Y, Ohno H, Saito T, Katayama T, Yagita H, Okumura K, et al. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity 1994; 1:357 - 64; http://dx.doi.org/10.1016/1074-7613(94)90066-3; PMID: 7533644
- Seki N, Brooks AD, Carter CR, Back TC, Parsoneault EM, Smyth MJ, Wiltrout RH, Sayers TJ. Tumor-specific CTL kill murine renal cancer cells using both perforin and Fas ligand-mediated lysis in vitro, but cause tumor regression in vivo in the absence of perforin. J Immunol 2002; 168:3484 - 92; PMID: 11907109
- Shimizu M, Takeda Y, Yagita H, Yoshimoto T, Matsuzawa A. Antitumor activity exhibited by Fas ligand (CD95L) overexpressed on lymphoid cells against Fas+ tumor cells. Cancer Immunol Immunother 1998; 47:143 - 8; http://dx.doi.org/10.1007/s002620050514; PMID: 9829839
- Caldwell SA, Ryan MH, McDuffie E, Abrams SI. The Fas/Fas ligand pathway is important for optimal tumor regression in a mouse model of CTL adoptive immunotherapy of experimental CMS4 lung metastases. J Immunol 2003; 171:2402 - 12; PMID: 12928387
- Dobrzanski MJ, Reome JB, Hollenbaugh JA, Hylind JC, Dutton RW. Effector cell-derived lymphotoxin alpha and Fas ligand, but not perforin, promote Tc1 and Tc2 effector cell-mediated tumor therapy in established pulmonary metastases. Cancer Res 2004; 64:406 - 14; http://dx.doi.org/10.1158/0008-5472.CAN-03-2580; PMID: 14729652
- Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, Hodge JW. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 2003; 170:6338 - 47; PMID: 12794167
- Braun MY, Lowin B, French L, Acha-Orbea H, Tschopp J. Cytotoxic T cells deficient in both functional fas ligand and perforin show residual cytolytic activity yet lose their capacity to induce lethal acute graft-versus-host disease. J Exp Med 1996; 183:657 - 61; http://dx.doi.org/10.1084/jem.183.2.657; PMID: 8627178
- Winter H, Hu HM, Urba WJ, Fox BA. Tumor regression after adoptive transfer of effector T cells is independent of perforin or Fas ligand (APO-1L/CD95L). J Immunol 1999; 163:4462 - 72; PMID: 10510388
- Lee SH, Bar-Haim E, Machlenkin A, Goldberger O, Volovitz I, Vadai E, Tzehoval E, Eisenbach L. In vivo rejection of tumor cells dependent on CD8 cells that kill independently of perforin and FasL. Cancer Gene Ther 2004; 11:237 - 48; http://dx.doi.org/10.1038/sj.cgt.7700678; PMID: 14739939
- Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, Blankenstein T. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res 2003; 63:4095 - 100; PMID: 12874012
- Böhm W, Thoma S, Leithäuser F, Möller P, Schirmbeck R, Reimann J. T cell-mediated, IFN-gamma-facilitated rejection of murine B16 melanomas. J Immunol 1998; 161:897 - 908; PMID: 9670968
- Wigginton JM, Gruys E, Geiselhart L, Subleski J, Komschlies KL, Park JW, Wiltrout TA, Nagashima K, Back TC, Wiltrout RH. IFN-gamma and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J Clin Invest 2001; 108:51 - 62; http://dx.doi.org/10.1172/JCI200110128; PMID: 11435457
- Kowalczyk DW, Wlazlo AP, Giles-Davis W, Kammer AR, Mukhopadhyay S, Ertl HC. Vaccine-induced CD8+ T cells eliminate tumors by a two-staged attack. Cancer Gene Ther 2003; 10:870 - 8; http://dx.doi.org/10.1038/sj.cgt.7700653; PMID: 14712313
- Barth RJ Jr., Mulé JJ, Spiess PJ, Rosenberg SA. Interferon gamma and tumor necrosis factor have a role in tumor regressions mediated by murine CD8+ tumor-infiltrating lymphocytes. J Exp Med 1991; 173:647 - 58; http://dx.doi.org/10.1084/jem.173.3.647; PMID: 1900079
- Prévost-Blondel A, Roth E, Rosenthal FM, Pircher H. Crucial role of TNF-alpha in CD8 T cell-mediated elimination of 3LL-A9 Lewis lung carcinoma cells in vivo. J Immunol 2000; 164:3645 - 51; PMID: 10725721
- Hollenbaugh JA, Reome J, Dobrzanski M, Dutton RW. The rate of the CD8-dependent initial reduction in tumor volume is not limited by contact-dependent perforin, Fas ligand, or TNF-mediated cytolysis. J Immunol 2004; 173:1738 - 43; PMID: 15265903
- Stalder T, Hahn S, Erb P. Fas antigen is the major target molecule for CD4+ T cell-mediated cytotoxicity. J Immunol 1994; 152:1127 - 33; PMID: 7507960
- Ju ST, Cui H, Panka DJ, Ettinger R, Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci U S A 1994; 91:4185 - 9; http://dx.doi.org/10.1073/pnas.91.10.4185; PMID: 7514297
- Listopad JJ, Kammertoens T, Anders K, Silkenstedt B, Willimsky G, Schmidt K, Kuehl AA, Loddenkemper C, Blankenstein T. Fas expression by tumor stroma is required for cancer eradication. Proc Natl Acad Sci U S A 2013; 110:2276 - 81; http://dx.doi.org/10.1073/pnas.1218295110; PMID: 23341634
- Trauth BC, Klas C, Peters AM, Matzku S, Möller P, Falk W, Debatin KM, Krammer PH. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 1989; 245:301 - 5; http://dx.doi.org/10.1126/science.2787530; PMID: 2787530
- Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med 1989; 169:1747 - 56; http://dx.doi.org/10.1084/jem.169.5.1747; PMID: 2469768
- Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature 1993; 364:806 - 9; http://dx.doi.org/10.1038/364806a0; PMID: 7689176
- Leontieva OV, Lenzo F, Demidenko ZN, Blagosklonny MV. Hyper-mitogenic drive coexists with mitotic incompetence in senescent cells. Cell Cycle 2012; 11:4642 - 9; http://dx.doi.org/10.4161/cc.22937; PMID: 23187803
- Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet 1995; 11:294 - 300; http://dx.doi.org/10.1038/ng1195-294; PMID: 7581453
- Karray S, Kress C, Cuvellier S, Hue-Beauvais C, Damotte D, Babinet C, Lévi-Strauss M. Complete loss of Fas ligand gene causes massive lymphoproliferation and early death, indicating a residual activity of gld allele. J Immunol 2004; 172:2118 - 25; PMID: 14764677
- Senju S, Negishi I, Motoyama N, Wang F, Nakayama K, Nakayama K, Lucas PJ, Hatakeyama S, Zhang Q, Yonehara S, et al. Functional significance of the Fas molecule in naive lymphocytes. Int Immunol 1996; 8:423 - 31; http://dx.doi.org/10.1093/intimm/8.3.423; PMID: 8671629
- Hao Z, Hampel B, Yagita H, Rajewsky K. T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. J Exp Med 2004; 199:1355 - 65; http://dx.doi.org/10.1084/jem.20032196; PMID: 15148335
- Peter ME, Legembre P, Barnhart BC. Does CD95 have tumor promoting activities?. Biochim Biophys Acta 2005; 1755:25 - 36; PMID: 15907590
- Eck MJ, Manley PW. The interplay of structural information and functional studies in kinase drug design: insights from BCR-Abl. Curr Opin Cell Biol 2009; 21:288 - 95; http://dx.doi.org/10.1016/j.ceb.2009.01.014; PMID: 19217274
- Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, Hirth P. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov 2012; 11:873 - 86; http://dx.doi.org/10.1038/nrd3847; PMID: 23060265