Keywords: :
Development is a highly ordered process that institutes considerable changes in cell cycle structure of the different cells composing the embryo. Cell fate is decided at early stages of embryogenesis in embryonic stem cells (ESCs). These cells are derived from the inner cell mass of the primordial embryo and can give rise to all cell lineages of the 3 primary germ layers. The cell cycle of ESCs is uncommonly rapid compared with a wide range of somatic cells due to shorter gap phases, especially G1, resulting in the characteristic high proportion of cells in S phase. Previous observations have highlighted the importance of a rapid and contracted cell cycle for ESCs maintenance. A high proliferation rate was shown to be required for maintenance of ESC identity,Citation1 and increased susceptibility to differentiate occurs during the contracted G1 phase.Citation2 Very recently, the molecular mechanism underlying cell fate decisions of self-renewing ESCs was analyzed in more detail and shown to originate at specific points within this critical retracted G1 phase,Citation3 establishing a connection between the mechanisms that control the cell cycle, cell fate decisions, and loss of pluripotency.
ESCs differentiation induces massive changes in the nuclear protein landscape, surprisingly reported to be largely regulated at the post-transcriptional level rather than by direct regulation of gene expression levels by transcription factors,Citation4 as previously postulated by various studies in diverse model organisms. One prominent protein post-translational modification is ubiquitylation, which regulates abundance and/or localization of target proteins altering their function, inasmuch regulating a multitude of cellular processes operating in most biological systems. Ubiquitylation is performed in 3 main consecutive steps catalyzed by E1, E2, and E3 enzymes, and in many cases the specificity of the reaction is provided by the E3 ligase complex, which conjugates activated ubiquitin to target substrates. The reverse reaction consists of removing ubiquitin from target proteins and is catalyzed by deubiquitylating enzymes (Dubs). Interestingly, converging evidences indicate that in ESCs, pluripotency is under tight control of the ubiquitin-dependent degradation pathways and proteasomal activity.Citation5,Citation6 In these studies, substrates and activity of the proteasome were shown to be different in ESCs compared with differentiated cells, reflecting differentiation-induced adaptations. For example, Nanog and Oct4, 2 key transcription factors involved in maintenance of pluripotency, were shown to have short half-lives in ESCs. Given that the activity of degradation pathways are commonly coupled to cell cycle stage and progression, it is not unlikely that both Nanog and Oct4 may be degraded in a cell cycle-dependent manner.
The ubiquitin proteasome degradation pathway governs major cell cycle events including G1 to S transition and anaphase. It is interesting to note that both in human and mouse ESCs, substrates of the anaphase-promoting complex/cyclosome (APC/C) have recently been identified as critical players in pluripotency maintenance.Citation3,Citation6 APC/C activity is elevated in late anaphase and persists through progression of the G1 phase. In human ESCs, Cyclin D levels are maximal at the G1/S transition and inhibit endoderm differentiation by inducing CDK4/6-dependent phosphorylation of Smad2 and Smad3 transcription factors, clearing them from chromatin and blocking expression of endoderm genes. In mouse ESCs, we have reported that post-translational control of Cdc25A, a cell cycle master regulator, by the Dub3 deubiquitylase, is essential for pluripotency maintenance by control of Cdc25A protein abundance. Indeed, the balance of ubiquitylating and deubiquitylating activities on Cdc25A is tipped toward deubiquitylation due to high levels of Dub3. In addition failure to reduce Dub3 levels during differentiation does not affect the onset of differentiation; however, it results in cell lethality at a time of cell cycle remodelling. This provides one molecular mechanism of how the cell cycle impacts cell fate decisions through contraction of the G1 phase and relaxation of the G1/S checkpoint.Citation6 In addition, the activity of SCF (Skip1/Cul1/F-box protein)-mediated degradation pathway, which directly controls abundance of 2 major cell cycle regulators, the p21 and p27 proteins, is itself under tight control of APC/CCdh1. Altogether, these observations emphasize the importance of the ubiquitin signaling pathways and associated Dub activities during development that remove ubiquitin from substrates and regulate proteasomal function.
These new observations tentatively provide insight into the regulation of midblastula transition onset in Drosophila, in which, similarly to mESCs, post-translational modifications of Cdc25A and not changes of its mRNA expression have also appeared as main determinants of cell cycle changes and fate decisions.Citation7 Altogether, these data converge to the concept that developmentally regulated, post-translational control of key cell cycle substrates and proteasomal activity are essential for regulating cell fate decisions of pluripotent stem cells and during development (). Given that the degradation pathway targeting substrates for degradation and associated proteasomal activity itself are cell cycle-dependent, it seems relevant to dissect the regulatory mechanisms that govern and institute these proteasome-dependent degradation pathways in an unperturbed pluripotent cell cycle.
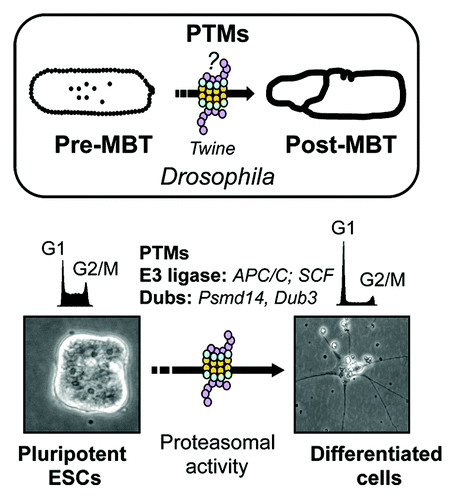
Figure 1. Schematic representation of developmental transitions mediated by protein post-translational modifications (PTMs). In Drosophila, PTMS are implicated in pre- to post-midblastula transition (MBT). The enzyme(s) responsible for Drosophila Cdc25 (twine) downregulation at MBT are unknown (question mark). In mouse embryonic stem cells (ESCs) PMTs change Cdc25A stability through Dub3 downregulation upon differentiation. Graphs represent cell cycle profiles.
References
- Ruiz S, et al. Curr Biol 2011; 21:45 - 52; http://dx.doi.org/10.1016/j.cub.2010.11.049; PMID: 21167714
- Sela Y, et al. Stem Cells 2012; 30:1097 - 108; http://dx.doi.org/10.1002/stem.1078; PMID: 22415928
- Pauklin S, et al. Cell 2013; 155:135 - 47; http://dx.doi.org/10.1016/j.cell.2013.08.031; PMID: 24074866
- Lu R, et al. Nature 2009; 462:358 - 62; http://dx.doi.org/10.1038/nature08575; PMID: 19924215
- Buckley SM, et al. Cell Stem Cell 2012; 11:783 - 98; http://dx.doi.org/10.1016/j.stem.2012.09.011; PMID: 23103054
- van der Laan S, et al. Mol Cell 2013; 52:366 - 79; http://dx.doi.org/10.1016/j.molcel.2013.10.003; PMID: 24207026
- Farrell JA, et al. Curr Biol 2013; 23:118 - 26; http://dx.doi.org/10.1016/j.cub.2012.11.036; PMID: 23290551
