Abstract
While cell proliferation is an essential part of embryonic development, cells within an embryo cannot proliferate freely. Instead, they must balance proliferation and other cellular events such as differentiation and morphogenesis throughout embryonic growth. Although the G1 phase has been a major focus of study in cell cycle control, it is becoming increasingly clear that G2 regulation also plays an essential role during embryonic development. Here we discuss the role of Cdc25, a key regulator of mitotic entry, with a focus on several recent examples that show how the precise control of Cdc25 activity and the G2/M transition are critical for different aspects of embryogenesis. We finish by discussing a promising technology that allows easy visualization of embryonic and adult cells potentially regulated at mitotic entry, permitting the rapid identification of other instances where the exit from G2 plays an essential role in development and tissue homeostasis.
After an egg is fertilized, the newly created zygote has an immediate need for cell proliferation. The single-celled zygote undergoes a period of generally rapid proliferation, and then the embryonic cells begin to specialize along different fate trajectories. The speed of these early divisions, as well as the number of divisions before specialization occurs, varies widely among species. However, after the early proliferative phase, all embryos need to balance cell division against other potentially conflicting developmental processes such as cell migration and differentiation.
One approach that embryonic cells use to overcome potential conflicts is temporally separating cell division from other aspects of development. Over 2 decades ago, hallmark work from Victoria Foe described domains of cells within the early fruit fly embryo (Drosophila melanogaster) that divide synchronously prior to major cell movement or differentiation.Citation1 Later work revealed that cell division is limited to specific times and locations by regulated transcription of a gene called string,Citation2 whose highly complex transcription is regulated by a multitude of cis regulatory elements spread over more than 30 kb of DNA.Citation3 String, an ortholog of the Saccharomyces pombe Cdc25,Citation4 is a primary regulator of mitotic entry. Interestingly, domains of dividing cells within the early Drosophila embryo are preceded by transcription of string,Citation2 and each of the mitotic domains were proposed to contain cells that would accept a unique cell fate.Citation1 Thus, this work collectively suggested a link between mitotic entry, the cell cycle, and differentiation.
Surprisingly, forced expression of string uniformly throughout a developing Drosophila embryo had little effect on embryonic development.Citation5 Researcher’s attention thus shifted to the need of the embryo to regulate string expression in order to permit specific cell migration events, which require key cytoskeletal elements also involved in mitosis.Citation6 For years, limiting demands on the cytoskeleton by halting mitosis during cell migration was considered the primary reason for regulating Cdc25 and thus controlling the timing of mitotic entry in embryos (see “Cdc25 in morphogenesis”). Recent studies, however, have uncovered additional roles for regulating production of Cdc25 within developing embryos from a variety of species. This review will explore a role for regulated Cdc25 activity in remodeling the cell cycle of the very early embryo, expand on the established role for limited Cdc25 during embryo morphogenesis, and consider new evidence for regulation of Cdc25 during differentiation. We close with an exciting new technology that promises to improve the identification of cells regulated at mitotic entry, which we expect will dramatically add to the list of cell types that limit Cdc25 for proper differentiation during embryonic development and elucidate the importance of G2 control in cycling cells.
The Cleavage Stages: A Special G2 at the First Cell Cycle
A primary function of a newly formed embryo is mass production of new cells during what is referred to as the cleavage stages of embryonic growth. Much of what is known about cleavage stage cell divisions come from the study of the frog Xenopus laevis, which are fecund external fertilizers with cells that are large and easy to see. Because cleavage stage cells are stationary and multipotent, differentiation and morphogenesis do not need to be integrated with division, and consequently the cell cycle is extremely rapid. During a prototypical cell cycle, the cell moves through mitosis (M) and DNA synthesis (S) with gap phases (G1 and G2) separating each. By contrast, these special cleavage stage embryonic cells alternate between M and S phases with no gap phases, and thus new cells are created as quickly as DNA can be replicated, and cell division can occur ().
Figure 1. Regulation of G2 during early embryogenesis. (A) The interphase of the first cell cycle is extended by a transient G2 phase, which is subsequently lost and regained several hours later during the midblastula transition (MBT). (B) Mitotic entry is controlled by heterodimerization of Cyclin B and Cdk1, and by the phosphorylation status of Cdk1, which is dictated by the ratio of Cdc25 to Wee1.
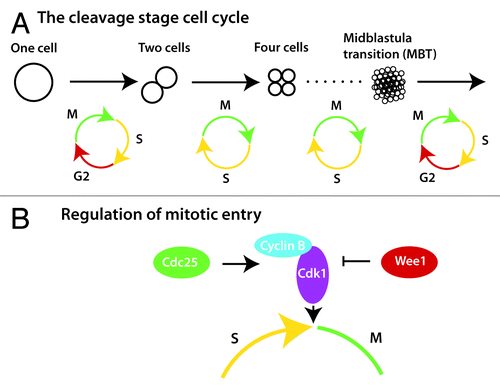
How is this early cell cycle controlled? In typical cells, the timing of the G2/M transition is regulated by the transcription of cyclin B during G2 and the subsequent degradation of Cyclin B at the end of M.Citation7 However, in cleavage stage frog embryos zygotic transcription has not yet begun. So how is a rapid cell cycle possible? The answer is that cyclin B mRNA is deposited in the egg, and alternating rounds of synthesis and destruction of Cyclin B protein from the maternally provided mRNA are sufficient to propel a cell through the cleavage stage cell cycle.Citation8 When embryos near the end of the cleavage stages, transcription begins, and the embryo can then make its own cyclin B mRNA to continue the cell cycle.
Cyclin B, in complex with cyclin-dependent kinase 1 (Cdk1), drives a phosphorylation cascade that moves cells into mitosis.Citation9 Cyclin B/Cdk1 complexes require more than heterodimerization to trigger mitotic entry. They also require a specific phosphorylation state for activation achieved by dephosphorylation of a specific tyrosine by the phosphatase Cdc25.Citation9 In the cleavage stage Xenopus embryo, Cdc25 from maternal deposits keeps Cyclin B/Cdk1 complexes in the active state.
Opposing the tyrosine phosphatase activity of Cdc25 is the tyrosine kinase Wee1, which inhibits activation of the Cyclin B/Cdk1 complexes.Citation9 Thus, the balance between Cdc25 and Wee1 mediate dephosphorylation and phosphorylation events that are critical for regulating the timing of mitotic entry (). For example, the first cell cycle in Xenopus is 3 times longer than the subsequent cell cycles (), and recent work has shown that modulating the balance between Cdc25 activity and Wee1 activity regulates the speed of the first cell cycle by creating a transient G2 phase ().Citation10 High initial levels of Wee1 relative to Cdc25 account for the slow first cell cycle, which is critical for embryo viability.Citation10 In Xenopus, the long, first cell cycle allows 2 key events to occur. First, it allows time for the male and female pronuclei to find each other and fuse, which is necessary for forming the diploid embryo. Second, it provides time for movement of special determinants from the bottom of the embryo to one side, creating the conditions for the formation of the anterior–posterior axis.Citation11 While the movement of determinants is only found in lower vertebrates such as fish and frogs, pronuclear fusion is a ubiquitous phenomenon. Intriguingly, a longer first cell cycle has also been observed in mice and humans,Citation12,Citation13 suggesting that a delayed first cell cycle is important for creating time to allow pronuclear fusion to occur correctly. It will be interesting to learn if the Wee1/Cdc25 ratio also regulates a delayed first cell cycle in vertebrates other than frogs.
The Midblastula Transition: Introducing Cell Cycle Complexity Through Regulation of Cdc25
After a fixed, organism-specific number of accelerated cleavage stage cycles, the cell cycle slows with the reacquisition of a G2 phase (). Because this change happens when the embryo is in an early multi-cellular state (the blastula) it is often called the midblastula transition (MBT), and is alternatively called the maternal to zygotic transition (MZT), because it coincides with the time when the embryo first begins to transcribe its own genes and thus switches from reliance on maternal factors to zygotic proteins produced from the embryo’s genome. The MBT has been a subject of interest for over 40 y, particularly with the seminal papers of Newport and Kirschner,Citation14,Citation15 both for its embryological importance and for its interesting aspects of cell cycle and transcriptional regulation.
Key to the acquisition of the G2 phase in all model systems studied to date is the regulation of Cdc25. Whereas Cdc25 levels are high in the egg and early embryo, at the MBT the levels sharply decrease. Early Drosophila embryos express 2 homologs of Cdc25, String and Twine.Citation16 Recent studies in Drosophila have shown that onset of the MBT is due to rapid degradation of the Cdc25 ortholog Twine at the precise time the MBT begins, with the half-life of Twine changing from 20 min to 5 min ().Citation17,Citation18 This rapid degradation, which introduces a cell cycle delay, is controlled by at least 2 factors, including a potential adaptor protein named Tribbles.Citation17,Citation19 But how does the embryo know precisely when to degrade Twine? Part of the answer involves transcription of Twine-degrading factors,Citation18 most likely regulated by Vielfältig/Zelda, the key transcriptional activator of the zygotic genome at the MBT.Citation20-Citation22 In addition, precise control of timing is determined by the ability of the embryo to measure the ratio of nuclear content to the amount of cytoplasm (the nucleocytoplasmic ratio). At each cell cycle the amount of DNA doubles, and at a set number of cycles, which differs among organisms, the embryo senses through a still unclear mechanism that a correct ratio has been reached, and this initiates the degradation of Twine.Citation17,Citation18
Figure 2. Regulation of the MBT onset by Cdc25. In Drosophila, zygotic transcription combined with attaining the correct nucleocytoplasmic (N/C) ratio triggers the acquisition of a G2 phase at the MBT. Production of Twine (Cdc25) degrading proteins, including Tribbles, causes rapid degradation of Twine. In addition, activation of Grapes (Chk1) leads to the degradation of String (a second Cdc25). In Xenopus and zebrafish, the correct N/C ratio triggers Chk1 activation, causing the degradation and inactivation of Cdc25 and the onset of G2.
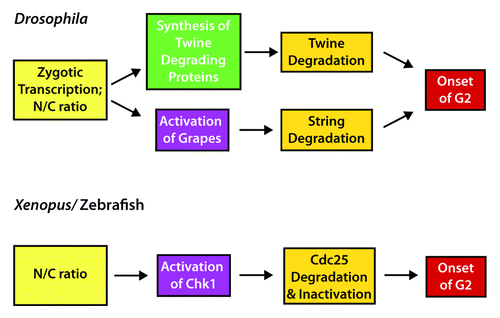
But Twine degradation is only part of the story. The other Cdc25 ortholog, String, also must be degraded for the MBT to occur. Like Twine, the String half-life also changes from 20 min to 5 min between the early cleavage embryo and the MBT, but this change is gradual and begins well before the MBT.Citation17,Citation18,Citation23 Key to this degradation is the Chk1 kinase ortholog Grapes,Citation24 which appears to require zygotic transcription to be activated ().Citation5 Although a pre-MBT requirement for zygotic gene expression seems to be at odds with the concept that the zygotic genome is not transcribed before the MBT, recent evidence shows that Vielfältig/Zelda can surprisingly transcribe many genes prior to the MBT.Citation21,Citation22,Citation25 But why does Grapes/Chk1 cause pre-MBT degradation of String but not Twine? Mutation of the consensus Chk1 phosphorylation sites in Twine does not affect its stability, demonstrating that String and Twine are not regulated the same way.Citation17 While this provides a satisfying mechanistic explanation of the regulation of the MBT in Drosophila, it is not clear why 2 Cdc25 factors are used in the early embryo, especially since Twine seems to be the key factor in timing G2 at the MBT.
In vertebrates, particularly the Xenopus and zebrafish, some aspects are conserved with Drosophila, and other features are different (). Similar to the regulation of Twine, Cdc25A is abruptly degraded at the MBT in Xenopus,Citation26 although as with String, the degradation is regulated by Chk1, which is transiently activated coincident with the start of the MBT.Citation27 In Xenopus, Cdc25A degradation alone does not affect the MBT, since overexpression of a stabilized Cdc25A does not change the cell cycle length at the MBT.Citation27,Citation28 However, phosphorylation of Cdc25A by Chk1 inactivates it, and this inactivation is essential for establishing the MBT.Citation28 Thus, both inactivation and degradation are likely to be involved in regulating Cdc25 in vertebrates (). Unlike in Drosophila, Chk1 phosphorylation and activation is independent of zygotic transcription in Xenopus.Citation14,Citation27 Instead, Chk1 activation depends on the depletion of specific DNA replication factors and limiting dNTP levels, which occurs at a precise nucleocytoplasmic ratio.Citation29
Although we now know quite a bit about the mechanism of the MBT in select model organisms, why does an MBT even exist? By executing a very fast cell cycle without transcription, embryos can quickly reach a multi-cellular stage. Thus, when transcription begins, it can be regionally controlled so that particular domains of the embryo can be specified to have particular cell fates. Although some transcription can occur in the pre-MBT embryo,Citation21,Citation25,Citation30,Citation31 a long interphase is presumably more conducive to optimal transcription, and so the slowing of the cell cycle is set to coincide with the onset of transcription.
Cdc25 and Morphogenesis
After the MBT, the cleavage stages of embryonic development are complete and cell differentiation begins. The initial stage of differentiation in multicellular organisms coincides with the major morphological event of gastrulation, in which the embryo essentially changes from a simple ball of cells into a multi-layered structure. As part of this process, several signaling cascades activate different differentiation pathways,Citation32 and many cells are subsequently induced to migrate. The specifics of the cell migration depend on the organism under consideration,Citation33 but the general theme is that migrating cells move to the inner layer of the embryo rather than remaining on the outside.
Cells that are moving from the outer layer to the inner layer of a gastrulating embryo typically undergo an epithelial-to-mesenchymal transition (EMT).Citation34 In a variety of cell types, EMT is commonly preceded by apical constriction, or shortening of a cell’s apical side by actin and myosin-mediated contraction ().Citation35 In gastrulating Drosophila and Xenopus embryos, cells undergoing apical constriction block cell division by limiting the activity of Cdc25. In Xenopus, this is accomplished by an increase in wee1 expression in the cells undergoing apical constriction.Citation36,Citation37 In Drosophila, Tribbles causes the degradation of the Cdc25 orthologs, String and Twine.Citation38-Citation40 If Cdc25 activity is not restricted in either model system, cells that should be gastrulating continue to divide, which places too much demand on the cytoskeleton eventually leading to embryo lethality.Citation6,Citation36,Citation38,Citation39 Gastrulating zebrafish cells induce an EMT that is actin-mediated but does not use apical constriction ().Citation33 Zebrafish embryos continue to express cdc25 within the cells that undergo gastrulation,Citation41 demonstrating that unrestricted Cdc25 does not affect gastrulation in all model systems, and instead depends on the type of cell movements that underlie the EMT.
Figure 3. Cell movements of gastrulation and neural tube formation (A) Gastrulation cell movements are commonly initiated by constriction of the apical surface of a cell (red). During gastrulation of zebrafish embryos, apical constriction is not used. Models are shown as cross-sections. (B) In Ciona, closure of the neural tube is controlled by actin-mediated elongation of cells in the epidermal cell layer (Ep). Elongating cells (outlined in green) provide the force that creates an internalized neural tube (NT). In both panels, cells to be internalized are blue, and cells to remain on the outside are yellow.
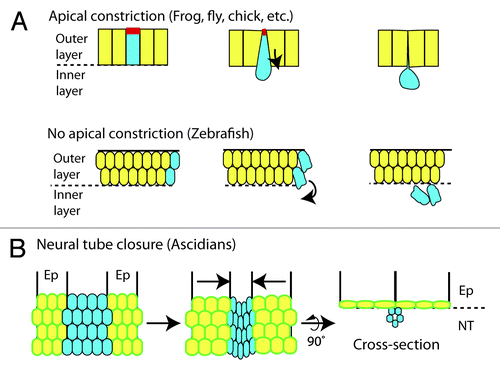
Another clear example where G2 regulation is necessary for morphogenesis is found in the sea squirt Ciona intestinalis, a model basal chordate, in which neural tube formation is similar to that of vertebrates. The neural tube forms from a sheet of cells that rolls up to form a hollow oblong cylinder that will become the spinal cord (). Attached on either side of the neural cells are epidermal cells that will cover the tube once it has formed. Just as the neural tube is rolling up, the proliferating epidermal cells prolong their G2 phase by regulating Cdc25.Citation42 If Cdc25 is overexpressed specifically in the epidermal cells, the G2 phase is shortened, and the neural tube fails to close.Citation42 During normal neural tube morphogenesis, the epidermal cells elongate in an actin-dependent manner, and this may provide the force that closes the neural tube ().Citation43 By prolonging G2 during this event, the epidermal cells can use their cytoskeleton for cell shape changes without the competing demands of mitosis. Although microtubules are an integral part of the cytoskeleton for cell shape and spindle fiber formation, these results suggest that restricting Cdc25 activity is required specifically in cells that rely heavily on actin and myosin for morphogenetic movements.
Cdc25 in Differentiation
After gastrulation is complete and cells have begun to differentiate, the anterior–posterior (AP) axis becomes increasingly more distinct. In vertebrates and many invertebrates, the AP axis forms through a process called posterior growth.Citation44 The head is established early, whereas the remainder of the body forms gradually by extending in the posterior direction until the complete AP axis is formed. In vertebrates, this is most clearly seen with the formation of the blocks of muscle cells called somites, which are formed and added to the AP axis in the posterior end of the embryo ().
Figure 4. Cdc25 in posterior body growth (A) The vertebrate posterior body grows from anterior to posterior with cells leaving the progenitor zone (red) at the most posterior end to become early muscle precursors (blue). Early muscle precursors continue to differentiate (orange) and are packaged into chevron-shaped blocks of cells called somites. Ultimately, the precursors will fuse together in the somites to become multi-nucleated muscle. (B) As cells move through the different stages of differentiation in zebrafish, they move through periods of low Cdc25 transcription (purple) and high Cdc25 transcription (green). The low periods of Cdc25 transcription are critical for differentiation to muscle.
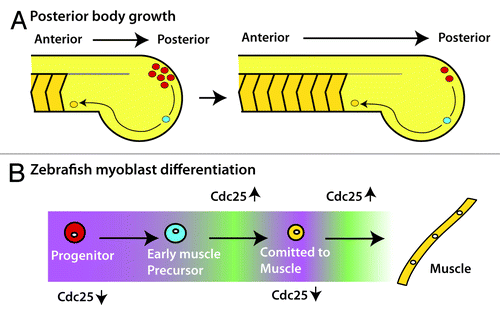
During vertebrate AP axis formation, stem cell-like progenitor cells, which can contribute to either muscle or neural tissue, drive the process of posterior growth.Citation45 Posterior progenitor cells are maintained in the hind end of the embryo and progressively leave the stem zone to contribute to the length of the body (). Recent work has established a clear connection between proliferation and the expression of cdc25a during posterior growth in zebrafish; intriguingly, the posterior progenitors were found to be cdc25a negative and quiescent.Citation41 As cells move through differentiation, 2 cycles of cdc25a expression are initiated, which, in turn, activates 2 rounds of cell division ().Citation41,Citation46 If Cdc25a is ubiquitously expressed in zebrafish embryos during posterior growth, development is severely perturbed.Citation41
Why is the cell cycle so tightly regulated during this process? Surprisingly, forced expression of Cdc25a prevented the normal differentiation of muscle cells, because the cells remained trapped in an early precursor state due to the aberrant maintenance of a key early differentiation gene that is normally only transiently expressed.Citation41,Citation47 Thus, regulated Cdc25 expression is essential for normal differentiation during posterior body growth and is involved in maintaining proper gene expression.
How does Cdc25 activity affect differentiation? An intriguing possibility is that since the progenitor cells are undergoing rapid changes in gene expression as they differentiate changes in the chromatin structure or epigenetic marks may require sufficient time before the next step in differentiation can begin. By forcing cells to progress through the cell cycle too quickly or at inappropriate times, cells may not be able to appropriately modify their DNA and thus remain stuck in an early phase of the differentiation process. Since the number of differentiating posterior progenitor cells in the zebrafish embryo is too few to test these ideas with current methodologies, identifying other sources of cells naturally held in G2 will be useful.
Future Directions
Why does the embryo occasionally use the G2/M transition instead of the well-studied G1/G0 phase as a place to halt the cell cycle? Because G1 is involved in sensing metabolic, environmental and stress cues,Citation48 it would seem logical for cells to halt in G1 at the start of the cell cycle, but the evidence discussed here has established that the G2/M transition is also an essential stopping point. The number of cases shown so far where cells are in a prolonged G2 state is small, and these have been found fortuitously, particularly in the very early embryo where these examples are easier to identify. While it is possible that these are the only situations where the G2/M transition is the key regulation point, we suggest that with improved methodology, additional examples will be found. With more examples to study, overarching principles that underlie G2/M regulation should become clear.
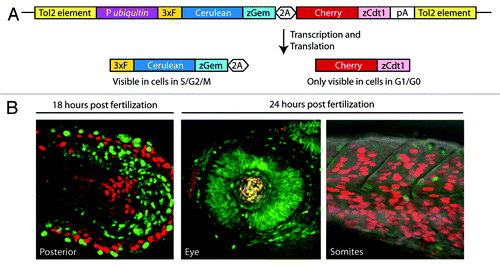
A recent technology called fluorescent ubiquitin-mediated cell cycle indicators (Fucci) has the ability to identify more cell types held in G2 in vivo. Fucci uses fluorescently labeled protein fragments that are differentially degraded as a live cell moves through the cell cycle.Citation49 In the latest incarnation, called Dual Fucci, a red G1/G0 indicator and a blue G2/S/M indicator are expressed from a single transcript in transgenic zebrafish embryos and adults using a ubiquitous promoter ().Citation41 Simply observing embryos, larvae or adults, under a fluorescent microscope, either alive or as fixed tissue sections, cells held in S/G2 (all blue), held in G1/G0 (all red), or cycling (mixed red and blue cells) can be identified (). Moreover, using a transgenic line that allows heat-inducible expression of Cdc25, it is possible to quickly examine if changing Cdc25 levels affects the cell cycle and fate of any candidate cells.Citation41 For example, the Fucci approach reveals that retinal cells of the early embryo are all in the S/G2/M state (),Citation49 and it will be worthwhile to examine the role of Cdc25 in regulating the cell cycle state of these cells.
Figure 5. The Fucci system aids identification of cells in G2 (A) The Dual Fucci transgene uses a zebrafish ubiquitin promoter (P ubiquitin) to drive expression of both of the components of the Fucci system from a single transgene throughout embryogenesis and into adulthood. A 3x FLAG tag (3xF) and a viral 2A peptide (2A) were used in this construct. (B) Using Dual Fucci and live or static imaging, clusters of cells held at the G2/M transition can be identified by the presence of the fluorescent protein Cerulean (pseudo-colored green in the included images) and the absence of Cherry.
Since Fucci was originally developed for use in the mouse,Citation50 the same approach can be used in this system, and likely in other animals as well, as with Ciona and zebrafish.Citation42,Citation49 One note of caution, it is important not to use promoters that express the Fucci at high levels, or cells will not degrade the fluorescent indicators or faithfully report the state of the cell cycle. Even with this caveat, Fucci promises the ability to identify new instances where cells are held in G2 in different animal systems at all stages of development and in all tissues.
Conclusions
Restricting the activity of Cdc25 and blocking the embryonic cell cycle before mitotic entry is a strategy that cells within a single embryo will use repeatedly during the early stages of embryonic development. Interestingly, Cdc25 can be controlled through a variety of mechanisms, including transcriptional control, phosphorylation, degradation of Cdc25 itself, and the use of the opposing kinase Wee1. Control at G2 contrasts with the well-studied G0/G1 regulation, and may be more widely used in embryos that face specific issues such as complex morphogenetic movements, a fast cell cycle, and rapid differentiation. We expect more examples of this type of cell cycle control will be found, particularly with broader use of the Fucci technology that allows the rapid visual identification of cells in a prolonged G2 phase. A larger collection of cell types for study will help us better understand the various reasons why G2/M is specifically used as an essential checkpoint.
| Abbreviations: | ||
| MBT | = | midblastula transition |
| MZT | = | maternal–zygotic transition |
| EMT | = | epithelial–mesenchymal transition |
| Fucci | = | fluorescent ubiquitin-mediated cell cycle indicators |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Alyssa Manning and Emily Mazanka for their thoughtful comments on the manuscript. This work was supported by an NIH grant (RO1GM079203) to D.K. and a Ruth Kirschstein NRSA fellowship (GM099306) to C.B.
References
- Foe VE. Mitotic domains reveal early commitment of cells in Drosophila embryos. Development 1989; 107:1 - 22; PMID: 2516798
- Edgar BA, O’Farrell PH. Genetic control of cell division patterns in the Drosophila embryo. Cell 1989; 57:177 - 87; http://dx.doi.org/10.1016/0092-8674(89)90183-9; PMID: 2702688
- Lehman DA, Patterson B, Johnston LA, Balzer T, Britton JS, Saint R, Edgar BA. Cis-regulatory elements of the mitotic regulator, string/Cdc25. Development 1999; 126:1793 - 803; PMID: 10101114
- Russell P, Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell 1986; 45:145 - 53; http://dx.doi.org/10.1016/0092-8674(86)90546-5; PMID: 3955656
- Edgar BA, O’Farrell PH. The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string. Cell 1990; 62:469 - 80; http://dx.doi.org/10.1016/0092-8674(90)90012-4; PMID: 2199063
- Foe VE, O’Dell GM, Edgar BA. Mitosis and morphogenesis in the Drosophila embryo: point and counterpoint. In: The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratories Press; 1993. page 149–300.
- Murray AW. Recycling the cell cycle: cyclins revisited. Cell 2004; 116:221 - 34; http://dx.doi.org/10.1016/S0092-8674(03)01080-8; PMID: 14744433
- Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature 1989; 339:275 - 80; http://dx.doi.org/10.1038/339275a0; PMID: 2566917
- Morgan DO. The Cell Cycle: Principles of control. London: New Science Press Ltd; 2007.
- Tsai TY-C, Theriot JA, Ferrell JE Jr.. Changes in oscillatory dynamics in the cell cycle of early Xenopus laevis embryos. PLoS Biol 2014; 12:e1001788; http://dx.doi.org/10.1371/journal.pbio.1001788; PMID: 24523664
- Weaver C, Kimelman D. Move it or lose it: axis specification in Xenopus. Development 2004; 131:3491 - 9; http://dx.doi.org/10.1242/dev.01284; PMID: 15262887
- Chen AA, Tan L, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril 2013; 99:1035 - 43; http://dx.doi.org/10.1016/j.fertnstert.2013.01.143; PMID: 23499001
- Ciemerych MA, Sicinski P. Cell cycle in mouse development. Oncogene 2005; 24:2877 - 98; http://dx.doi.org/10.1038/sj.onc.1208608; PMID: 15838522
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell 1982; 30:675 - 86; http://dx.doi.org/10.1016/0092-8674(82)90272-0; PMID: 6183003
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell 1982; 30:687 - 96; http://dx.doi.org/10.1016/0092-8674(82)90273-2; PMID: 7139712
- Edgar BA, Datar SA. Zygotic degradation of two maternal Cdc25 mRNAs terminates Drosophila’s early cell cycle program. Genes Dev 1996; 10:1966 - 77; http://dx.doi.org/10.1101/gad.10.15.1966; PMID: 8756353
- Di Talia S, She R, Blythe SA, Lu X, Zhang QF, Wieschaus EF. Posttranslational control of Cdc25 degradation terminates Drosophila’s early cell-cycle program. Curr Biol 2013; 23:127 - 32; http://dx.doi.org/10.1016/j.cub.2012.11.029; PMID: 23290553
- Farrell JA, O’Farrell PH. Mechanism and regulation of Cdc25/Twine protein destruction in embryonic cell-cycle remodeling. Curr Biol 2013; 23:118 - 26; http://dx.doi.org/10.1016/j.cub.2012.11.036; PMID: 23290551
- Lohan F, Keeshan K. The functionally diverse roles of tribbles. Biochem Soc Trans 2013; 41:1096 - 100; http://dx.doi.org/10.1042/BST20130105; PMID: 23863185
- Sung HW, Spangenberg S, Vogt N, Großhans J. Number of nuclear divisions in the Drosophila blastoderm controlled by onset of zygotic transcription. Curr Biol 2013; 23:133 - 8; http://dx.doi.org/10.1016/j.cub.2012.12.013; PMID: 23290555
- Liang H-L, Nien C-Y, Liu H-Y, Metzstein MM, Kirov N, Rushlow C. The zinc-finger protein Zelda is a key activator of the early zygotic genome in Drosophila. Nature 2008; 456:400 - 3; http://dx.doi.org/10.1038/nature07388; PMID: 18931655
- Harrison MM, Li X-Y, Kaplan T, Botchan MR, Eisen MB. Zelda binding in the early Drosophila melanogaster embryo marks regions subsequently activated at the maternal-to-zygotic transition. PLoS Genet 2011; 7:e1002266; http://dx.doi.org/10.1371/journal.pgen.1002266; PMID: 22028662
- Edgar BA, Sprenger F, Duronio RJ, Leopold P, O’Farrell PH. Distinct molecular mechanism regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes Dev 1994; 8:440 - 52; http://dx.doi.org/10.1101/gad.8.4.440; PMID: 7510257
- Sibon OC, Stevenson VA, Theurkauf WE. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature 1997; 388:93 - 7; http://dx.doi.org/10.1038/40439; PMID: 9214509
- Pritchard DK, Schubiger G. Activation of transcription in Drosophila embryos is a gradual process mediated by the nucleocytoplasmic ratio. Genes Dev 1996; 10:1131 - 42; http://dx.doi.org/10.1101/gad.10.9.1131; PMID: 8654928
- Kim SH, Li C, Maller JL. A maternal form of the phosphatase Cdc25A regulates early embryonic cell cycles in Xenopus laevis. Dev Biol 1999; 212:381 - 91; http://dx.doi.org/10.1006/dbio.1999.9361; PMID: 10433828
- Shimuta K, Nakajo N, Uto K, Hayano Y, Okazaki K, Sagata N. Chk1 is activated transiently and targets Cdc25A for degradation at the Xenopus midblastula transition. EMBO J 2002; 21:3694 - 703; http://dx.doi.org/10.1093/emboj/cdf357; PMID: 12110582
- Uto K, Inoue D, Shimuta K, Nakajo N, Sagata N. Chk1, but not Chk2, inhibits Cdc25 phosphatases by a novel common mechanism. EMBO J 2004; 23:3386 - 96; http://dx.doi.org/10.1038/sj.emboj.7600328; PMID: 15272308
- Collart C, Allen GE, Bradshaw CR, Smith JC, Zegerman P. Titration of four replication factors is essential for the Xenopus laevis midblastula transition. Science 2013; 341:893 - 6; http://dx.doi.org/10.1126/science.1241530; PMID: 23907533
- Kimelman D, Kirschner M, Scherson T. The events of the midblastula transition in Xenopus are regulated by changes in the cell cycle. Cell 1987; 48:399 - 407; http://dx.doi.org/10.1016/0092-8674(87)90191-7; PMID: 3802197
- Skirkanich J, Luxardi G, Yang J, Kodjabachian L, Klein PS. An essential role for transcription before the MBT in Xenopus laevis. Dev Biol 2011; 357:478 - 91; http://dx.doi.org/10.1016/j.ydbio.2011.06.010; PMID: 21741375
- Kimelman D. Mesoderm induction: from caps to chips. Nat Rev Genet 2006; 7:360 - 72; http://dx.doi.org/10.1038/nrg1837; PMID: 16619051
- Solnica-Krezel L, Sepich DS. Gastrulation: making and shaping germ layers. Annu Rev Cell Dev Biol 2012; 28:687 - 717; http://dx.doi.org/10.1146/annurev-cellbio-092910-154043; PMID: 22804578
- Keller R, Davidson L. Cell movements of gastrulation. In: Gastrulation: From Cells to Embryo. Cold Spring Harbor, NY: Cold Spring Harbor Laboratories Press; 2004. pp 291–304.
- Martin AC, Goldstein B. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development 2014; 141:1987 - 98; http://dx.doi.org/10.1242/dev.102228; PMID: 24803648
- Murakami MS, Moody SA, Daar IO, Morrison DK. Morphogenesis during Xenopus gastrulation requires Wee1-mediated inhibition of cell proliferation. Development 2004; 131:571 - 80; http://dx.doi.org/10.1242/dev.00971; PMID: 14711880
- Leise W 3rd, Mueller PR. Multiple Cdk1 inhibitory kinases regulate the cell cycle during development. Dev Biol 2002; 249:156 - 73; http://dx.doi.org/10.1006/dbio.2002.0743; PMID: 12217326
- Mata J, Curado S, Ephrussi A, Rørth P. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell 2000; 101:511 - 22; http://dx.doi.org/10.1016/S0092-8674(00)80861-2; PMID: 10850493
- Grosshans J, Wieschaus E. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell 2000; 101:523 - 31; http://dx.doi.org/10.1016/S0092-8674(00)80862-4; PMID: 10850494
- Seher TC, Leptin M. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr Biol 2000; 10:623 - 9; http://dx.doi.org/10.1016/S0960-9822(00)00502-9; PMID: 10837248
- Bouldin CM, Snelson CD, Farr GH 3rd, Kimelman D. Restricted expression of cdc25a in the tailbud is essential for formation of the zebrafish posterior body. Genes Dev 2014; 28:384 - 95; http://dx.doi.org/10.1101/gad.233577.113; PMID: 24478331
- Ogura Y, Sakaue-Sawano A, Nakagawa M, Satoh N, Miyawaki A, Sasakura Y. Coordination of mitosis and morphogenesis: role of a prolonged G2 phase during chordate neurulation. Development 2011; 138:577 - 87; http://dx.doi.org/10.1242/dev.053132; PMID: 21205801
- Sasakura Y, Mita K, Ogura Y, Horie T. Ascidians as excellent chordate models for studying the development of the nervous system during embryogenesis and metamorphosis. Dev Growth Differ 2012; 54:420 - 37; http://dx.doi.org/10.1111/j.1440-169X.2012.01343.x; PMID: 22524611
- Kimelman D, Martin BL. Anterior-posterior patterning in early development: three strategies. Wiley Interdiscip Rev Dev Biol 2012; 1:253 - 66; http://dx.doi.org/10.1002/wdev.25; PMID: 23801439
- Wilson V, Olivera-Martinez I, Storey KG. Stem cells, signals and vertebrate body axis extension. Development 2009; 136:1591 - 604; http://dx.doi.org/10.1242/dev.021246; PMID: 19395637
- Nogare DE, Arguello A, Sazer S, Lane ME. Zebrafish cdc25a is expressed during early development and limiting for post-blastoderm cell cycle progression. Dev Dyn 2007; 236:3427 - 35; http://dx.doi.org/10.1002/dvdy.21363; PMID: 17969147
- Griffin KJ, Kimelman D. One-Eyed Pinhead and Spadetail are essential for heart and somite formation. Nat Cell Biol 2002; 4:821 - 5; http://dx.doi.org/10.1038/ncb862; PMID: 12360294
- Massagué J. G1 cell-cycle control and cancer. Nature 2004; 432:298 - 306; http://dx.doi.org/10.1038/nature03094; PMID: 15549091
- Sugiyama M, Sakaue-Sawano A, Iimura T, Fukami K, Kitaguchi T, Kawakami K, Okamoto H, Higashijima S, Miyawaki A. Illuminating cell-cycle progression in the developing zebrafish embryo. Proc Natl Acad Sci U S A 2009; 106:20812 - 7; http://dx.doi.org/10.1073/pnas.0906464106; PMID: 19923430
- Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 2008; 132:487 - 98; http://dx.doi.org/10.1016/j.cell.2007.12.033; PMID: 18267078
