The term epithelial to mesenchymal transition (EMT) designs the conversion of an epithelial cell in another cell with a fibroblastic-like shape. Besides being essential for embryo morphogenesis, EMT also provides tumoral cells with higher invasion and greater resistance to apoptosis and is related to the initial stages of tumor metastasis. Among the many mesenchymal genes induced during EMT, Snail1 has received a great attention since it is rapidly upregulated at the onset of the process and is required for its completion.Citation1 EMT is induced by several cytokines and growth factors being TGF-β that with a more general effect. Actually, Snail1 is rapidly induced by TGF-β not only in epithelial but also in mesenchymal cells and is required for a full response to this cytokine including the activation of invasion-related genes.Citation2 The article by Thakur et al.Citation3 in this issue of Cell Cycle has investigated the mechanism of activation of Snail1 transcription by TGF-β.
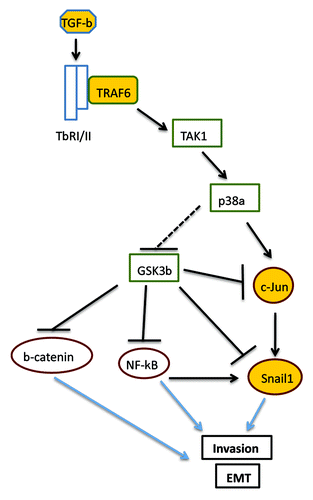
The TGF-β pathway includes Smad proteins that get activated by the TGF-β receptor and accumulate on chromatin to regulate gene expression.Citation4 In parallel, TGF-β receptors activate various protein kinases by recruiting directly adaptor proteins such as Shc and ubiquitin ligases such as TRAF6. Among these protein kinases, TGF-β activates TAK1 and the downstream effector p38 MAPK, at focus in the paper by Thakur et al.Citation3 This pathway is now shown to contribute via specific mechanisms to multiple physiological outcomes of TGF-β biology. Specifically, the TRAF6/TAK1 module provides transcriptional inputs to various gene targets, such as Snail1, that triggers cell invasion, and the cyclin-dependent kinase inhibitor p21 that feeds the cytostatic program and other pro-apoptotic genes.Citation3 Interestingly, the TGF-β/TRAF6/TAK1 pathway shows selectivity and Thakur et al. report on genes (e.g., Smad7) whose expression is not affected by this signaling module. The key open question in the TGF-β field has been the identification of transcription factors providing gene specificity. In this report the authors identify c-Jun as a TGF-β-dependent direct transcriptional activator of Snai1 gene.
These results have several implications and open new lines of future research. For instance, the dual role of TGF-β on c-Jun, first inducing its phosphorylation on Ser63 and its rapid activation and later its upregulation, might influence Snail1 expression in a two-wave fashion: first promoting an acute response required for triggering the EMT, and later a more sustained one, involved in reinforcing the phenotype and associated to a Jun-dependent extensive activation of genes required for invasion. It remains to be established how general this later response is because in other cells Snail1 activation by TGF-β is transient. Anyhow, the new model supports the idea that the molecular elements acting on EMT signaling pathways, such as p38α in this case, do not control a single but several steps.Citation1
The authors also show that TGF-β decreases GSK3β activity by phosphorylating this enzyme on Ser9. It remains to be established if this phosphorylation is mediated by the protein kinase that normally acts on this substrate, Akt and if so, how activation of Akt is performed by TGF-β. GSK3β inactivation is crucial for EMT since it opposes to Snail1 activation acting at different levels: transcriptionally, preventing not only c-Jun but also NF-κB binding to Snai1 promoter; and (2) de-stabilizing Snail1 protein.Citation1 Moreover, GSK3-β inhibition also enhances the stability and transcriptional function of β-catenin, an activity upregulated in migrating and invasive tumoral cells ().
Figure 1. Schema of TGF-β-induced activation of Snail1 expression. Indirect functional actions are indicated by dashed lines. Protein kinases are shown as rectangles and transcription factors as ovals. Colored molecules indicate those most rigorously investigated in the present study. See text for details.
Recent findings implicate yet another member of the TRAF ubiquitin ligase family, TRAF4, downstream of TGF-β receptor signaling.Citation5 It will be exciting to ascertain whether TRAF4 also uses the Jun-dependent and GSK3β-inhibitory mechanism to regulate Snail1. Furthermore, since TGF-β regulates a cohort of pro-invasive and metastatic genes using the Smad and activating protein 1 (AP1, i.e., Jun-Fos family) transcriptional complexes,Citation6 it will be of great interest to determine the impact of TRAF6/TAK1/Jun kinase pathway on the regulation of specific matrix metalloprotease genes. The latter is of therapeutic importance as recent studies on lung fibrosis identified the compound methacyclin that perturbs only TGF-β protein kinase but not canonical Smad signaling,Citation7 and thus provides a novel therapeutic opportunity for this class of chronic diseases. Implementation of such findings in cancer, including prostate, will be of essence.
References
- García de Herreros A, et al. Reviews on Cancer 2012; 1825:223 - 228; http://dx.doi.org/10.1016/j.bbcan.2012.01.003
- Batlle R, et al. Oncogene 2013; 32:3381 - 9; http://dx.doi.org/10.1038/onc.2012.342; PMID: 22869142
- Thakur N, et al. Cell Cycle 2014; 13:2400 - 14; http://dx.doi.org/10.4161/cc.29339; PMID: 24945502
- Moustakas A, et al. Semin Cancer Biol 2012; 22:446 - 54; http://dx.doi.org/10.1016/j.semcancer.2012.04.002; PMID: 22548724
- Zhang L, et al. Mol Cell 2013; 51:559 - 72; http://dx.doi.org/10.1016/j.molcel.2013.07.014; PMID: 23973329
- Sundqvist A, et al. Oncogene 2013; 32:3606 - 15; http://dx.doi.org/10.1038/onc.2012.370; PMID: 22926518
- Xi Y, et al. Am J Respir Cell Mol Biol 2014; 50:51 - 60; PMID: 23944988
