Abstract
The coordinated process of DNA replication and nucleosome assembly, termed replication-coupled (RC) nucleosome assembly, is important for the maintenance of genome integrity. Loss of genome integrity is linked to aging and cancer. RC nucleosome assembly involves deposition of histone H3-H4 by the histone chaperones CAF-1, Rtt106 and Asf1 onto newly-replicated DNA. Coordinated actions of these three histone chaperones are regulated by modifications on the histone proteins. One such modification is histone H3 lysine 56 acetylation (H3K56Ac), a mark of newly-synthesized histone H3 that regulates the interaction between H3-H4 and the histone chaperones CAF-1 and Rtt106 following DNA replication and DNA repair. Recently, we have shown that the lysine acetyltransferase Gcn5 and H3 N-terminal tail lysine acetylation also regulates the interaction between H3-H4 and CAF-1 to promote the deposition of newly-synthesized histones. Genetic studies indicate that Gcn5 and Rtt109, the H3K56Ac lysine acetyltransferase, function in parallel to maintain genome stability. Utilizing synthetic genetic array analysis, we set out to identify additional genes that function in parallel with Gcn5 in response to DNA damage. We summarize here the role of Gcn5 in nucleosome assembly and suggest that Gcn5 impacts genome integrity via multiple mechanisms, including nucleosome assembly.
Nucleosome Assembly Following DNA Replication and DNA Repair
Chromatin structure plays a significant role in several cellular processes including DNA replication, transcription and DNA repair.Citation1,Citation2 The fundamental unit of chromatin is the nucleosome which consists of 147 base pairs of DNA wrapped around the histone octamer containing two copies each of the core histones H2A, H2B, H3, and H4. During DNA replication, nucleosomes ahead of the replication fork are disassembled to allow DNA replication machinery access to the DNA. Immediately following DNA synthesis, replicated DNA must be assembled into nucleosomes and subsequent higher order chromatin structure. This process of coordinated DNA replication and nucleosome assembly, referred to as DNA replication-coupled (RC) nucleosome assembly,Citation3,Citation4 is important for epigenetic inheritance and the maintenance of genome integrity.Citation1,Citation2,Citation5 Loss of genome integrity is one of the key characteristics of cancer cells and aging.Citation6,Citation7 Therefore, understanding how RC nucleosome assembly is regulated is extremely important.
There are two pools of histones used for formation of nucleosomes following DNA replication: parental and newly-synthesized histones. While it is clear that assembly of newly-synthesized H3–H4 requires histone chaperones, it is not clear how parental histones are assembled. Histone chaperones are key proteins involved in the regulation of nucleosome assembly. By binding to histones, these proteins help shield the positively-charged his-tones from the negatively-charged DNA and facilitate histone deposition as well as histone removal.Citation2 In the yeast S. cerevisiae, the H3–H4 histone chaperones include Asf1, CAF-1 and Rtt106. These three his-tone chaperones function coordinately to efficiently deposit newly-synthesized his-tones onto the replicating DNA.Citation2,Citation8
Following repair of damaged DNA, nucleosomes must be reassembled and chromatin structure restored. This process is proposed to utilize similar machinery as replication-coupled nucleosome assembly. The current model to explain the role of chromatin structure and chromatin-associated proteins following DNA repair is the access-repair-restore model.Citation9,Citation10 Following DNA damage, repair factors must gain access to the DNA. Chromatin remodeling factors are involved in remodeling and/or disassembly of nucleosomes. Following repair of the lesion, nucleosomes and the chromatin structure must be reestablished. The histone chaperone CAF-1 is important for repair following DNA damage in both yeast and mammalian cells.Citation11–Citation13 In addition, the histone chaperone Asf1 is also linked to checkpoint activation associated with DNA damage through an interaction with Rad53.Citation14 Given that many of the same factors are involved, it is probable that many of the same regulatory mechanisms also control nucleosome assembly following DNA repair.
Multiple Acetylation Events Have Been Proposed to Regulate Nucleosome Assembly.
Newly-synthesized histones are associated with particular post-translational modifications that are thought to aid in the recognition and deposition of these histones at the replication fork.Citation2 Lysine acetylation is the most well-studied histone modification involved in the regulation of nucleosome assembly. Acetylation occurs prior to deposition, and the histones are deacetylated following deposition.Citation15 It has been known for a long time that the most highly conserved marks of newly-synthesized his-tones are acetylation of histone H4 lysine residues 5 and 12 (H4K5,12Ac).Citation16 These marks are catalyzed by Hat1.Citation17,Citation18 While this pattern is found on H4 in complex with CAF-1, drosophila and mammalian Asf1, and yeast Hif1,Citation17,Citation19–Citation21 the precise role of this mark in nucleosome assembly remains to be determined.
In yeast, newly-synthesized histone H3 is marked by lysine 56 acetylation (H3K56Ac).Citation22 This mark, catalyzed by the lysine acetyltransferase Rtt109, is found within the core domain of H3 and is dependent on the histone chaperone Asf1.Citation23–Citation26 H3K56Ac mediates the interaction between H3-H4 and CAF-1 and Rtt106, thereby promoting replication-coupled nucleosome assembly.Citation27 In yeast, it has been found that H3K56Ac helps drive chromatin assembly following repair and signals the completion of repair.Citation28 In mammalian cells, H3K56Ac has been found to have a role in DNA damage and repair. In addition, H3K56Ac has been associated with tumorigenicity, consistent with a role in genomic stability.Citation29,Citation30 The histone acetyltransferases responsible for H3K56ac in mammalian cells include CBP/p300 and Gcn5.Citation29,Citation31 Further studies are needed to fully understand the function of H3K56Ac in mammalian cells.
In addition to H3K56Ac, acetylation of H3 N-terminal tails has also been reported. However, patterns of acetylation of the N-terminus of newly-synthesized H3 are not as well conserved.Citation16 For instance, in yeast cells, newly-synthesized H3 is acetylated predominantly at lysine 9 followed by lysine 27.Citation16,Citation32 In mammalian cells, acetylation of H3.1, the H3 variant incorporated into chromatin during DNA replication, is barely detectable.Citation16 However, a recent study found that H3 lysine 14 and 18 are present on 20–30% of H3.1 in association with Asf1 during S-phase in HeLa cells.Citation21 Given the low conservation among species, the role of acetylated lysine residues at the N-terminus of H3 in nucleosome assembly is not well studied. Furthermore, the lysine acetyltransferase that acetylates the H3-N-terminus and functions in nucleosome assembly, until recently, was not known.
Recent studies from our laboratory suggest that the histone acetyltransferases Elp3 and Gcn5 are involved in marking newly-synthesized histones and regulating nucleosome assembly.Citation33,Citation34 Here, we review the role of Gcn5 in replication-coupled nucleosome assembly following DNA replication. In addition, we report the results of a synthetic genetic array (SGA) screen performed to identify genes that function in parallel to Gcn5 in response to DNA damage. We found that multiple pathways function in parallel with Gcn5 in the maintenance of genome integrity.
A Role for the Lysine Acetyltransferase Gcn5 in Replication-Coupled Nucleosome Assembly
Gcn5 (general control non-derepressible 5) was originally identified in a screen for factors involved in the regulation of amino acid biogenesis.Citation35,Citation36 Later studies showed that Gcn5 is a transcription activatorCitation37 and has lysine acetyltransferase activity and that this activity contributes towards its role in transcriptional activation.Citation38,Citation39 Gcn5 is highly conserved and is a catalytic component of several large complexes within yeast and higher eukaryotes.Citation40–Citation42 Gcn5 by itself has limited histone lysine acetyltransferase activity, namely acetylation of histone H3K14 as well as H4 K8 and 16 of free histones.Citation32 This activity is greatly expanded when Gcn5 is part of the larger ADA and SAGA complexes, which enable acetylation of nucleosomal histones.Citation43 Besides their shared components, both the ADA and SAGA complexes regulate gene transcription, in part, through other components of these complexes.Citation42,Citation44 For instance, Spt8, a unique component of the SAGA complex is involved in recruitment of TBP to active promoters, as is the component Spt3.Citation45,Citation46 In addition, the Sus1-Sgf11-Ubp8 ubiquitin complex of SAGA is involved in H2B deubiquitination as well as regulating the level of H3K4me3.Citation47–Citation50 While Gcn5 and its complex members' roles in transcriptional activation are well established, it has recently become clear that Gcn5 has roles beyond transcriptional regulation.
Cells that lack Gcn5 are sensitive to DNA damaging agents and exhibit G2/M arrest, suggesting that Gcn5 has a role in the maintenance of genome integrity.Citation51 While Gcn5′s role in transcription may help explain some of these phenotypes, it certainly doesn't explain them completely, therefore, suggesting that Gcn5 and histone N-terminal tail acetylation functions in processes besides gene transcription.Citation52 Our laboratory and others found that the histone H3K56 acetyltransferase, RTT109, exhibits a significant genetic interaction with GCN5 and H3 N-terminal tail acetylation in growth and DNA damage sensitivity, indicating that Gcn5 and H3 N-terminal tail acetylation function in parallel with H3K56Ac.Citation27,Citation34,Citation53 Compared to wild-type cells, both rtt109Δ and gcn5Δ mutant cells exhibit a higher percentage of Rad52 foci, a measure of spontaneous DNA damage. In addition, GCN5 exhibits a synthetic genetic interaction with genes encoding checkpoint kinases (RAD53 and MEC1), DNA replication proteins (CDC17 and CDC7), and histone chaperones (CAF-1, RTT106, and ASF1). These observations suggest that Gcn5′s role in the maintenance of genome integrity may lie, at least partly, in a role in DNA replication-coupled nucleosome assembly.Citation34 Supporting this idea, we have shown that the deposition of newly-synthesized H3, marked by H3K56Ac, is compromised in cells lacking Gcn5 or mutant cells unable to acetylate the N-terminus of H3. Furthermore, Gcn5 and N-terminal tail acetylation promote the interaction between H3-H4 and CAF-1.Citation34 These results provide strong support for the idea that Gcn5 has a role in replication-coupled nucleosome assembly, in part, through the acetylation of lysine residues at the H3 N-terminus.
Interestingly, while genetic evidence suggests that GCN5 and RTT109 function in parallel in response to DNA damage, our studies suggest a crosstalk between Gcn5 and Rtt109 during replication-coupled nucleosome assembly. H3K56Ac, catalyzed by Rtt109, increases the binding of H3–H4 with both Rtt106 and CAF-1.Citation27 In contrast, Gcn5 and acetylation of lysine residues at the H3 N-terminus impact the binding of CAF-1 to H3–H4 and have little impact on the association of Rtt106 with H3–H4. Moreover, Rtt109 can also acetylate lysine 9 and lysine 27 of H3 N-terminus, two residues that can also be acetylated by Gcn5.Citation34,Citation54 However, it is important to note that Gcn5, but not Rtt109, is the predominant acetyltransferase for these two lysine residues. In addition, while it is reported that Gcn5 can acetylate H3 K56 in mammalian cells,Citation31 in budding yeast, deletion of GCN5 has no apparent effect on H3K56Ac in cells.Citation34 These results suggest that although there is crosstalk between the two enzymes, they predominantly function in parallel pathways to regulate nucleosome assembly and maintain genome stability. Thus, Gcn5 and Rtt109 function together to promote nucleosome assembly and genome stability ().
Roles for Gcn5 in the Initiation of DNA Replication and Repair
Mutant gcn5Δ cells are significantly more sensitive to the DNA damaging agents hydroxyurea (HU), camptothecin (CPT), and methyl methanesulfonate (MMS) than cells lacking CAC1, the large subunit of CAF-1.Citation34 This suggests that Gcn5 (and furthermore, H3 N-terminal tail acetylation) may have functions beyond its role in regulating the interaction between CAF-1 and H3–H4 that contribute towards its general function in response to DNA damaging agents.Citation34 Our lab and others have found that Gcn5 also directly associates with origins of DNA replication. Moreover, cells lacking Gcn5 have a deficiency in the assembly of pre-replicative complexes and a significant disruption of the chromatin structure surrounding the replication origin.Citation55 These results suggest that Gcn5 has a role in the initiation of DNA replication.Citation34,Citation55 It is thought that Gcn5 may serve to decondense the chromatin structure to facilitate access for DNA replication machinery.Citation55 Supporting this idea, it has been found that histone N-terminal tail acetylation positively regulates origin firing in yeast cellsCitation55,Citation56 and that acetylation of multiple sites on H3 and H4 regulates origin firing.Citation56 Interestingly, acetylation of many of these same sites has been implicated or shown to have roles in replication-coupled nucleosome assembly. Because defects in nucleosome assembly can impact the integrity of chromatin structure, the observed defects in the initiation of DNA replication in cells lacking Gcn5 could be partly due to an indirect effect arising from defects in nucleosome assembly.
In mammalian cells, it's been found that Gcn5 acetylates Cdc6, a component of the pre-replication complex, stimulating its phosphorylation and activation.Citation57 While this interaction has not yet been observed in yeast, it is still possible that Gcn5 also contributes to origin firing by acetylating proteins involved in the initiation of DNA replication. Future studies are needed to address how Gcn5 regulates the initiation of DNA replication.
Gcn5 is also implicated in DNA repair. In yeast, Gcn5 is recruited to double strand break sites, and gcn5Δ cells are sensitive to DNA double strand breaks. In conjunction with a role for Gcn5 in double strand break repair, acetylation of multiple sites of the H3 N-terminus increases following a double strand break.Citation58 In addition, it has been shown that acetylation of H3 and Hat1, which catalyzes acetylation of lysine 5 and 12 of H4, is important for double strand break repair.Citation59 In mammalian cells, the STAGA and TFTC complexes, two of the large Gcn5-containing complexes in mammalian cells, are involved in UV damage repair.Citation60,Citation61 Despite these advances, it is still unclear how Gcn5 and acetylation of lysine residues at the H3-N-terminus function in DNA repair.
Given that Gcn5 and Rtt109 function in parallel in the response to DNA damaging agents, and Rtt109 promotes nucleosome assembly following both DNA replication and DNA repair,Citation27,Citation28,Citation34 it is possible that Gcn5 and acetylation of lysine residues of the H3 N-terminus function in DNA repair by promoting assembly of replicated DNA into nucleosomes. More recently, it has been shown that H3 N-terminal tail acetylation by Gcn5 following DNA damage may aid in the recruitment of the chromatin remodeling SWI/SNF complex, facilitating DNA damage signaling and repair.Citation62 Thus, Gcn5 and acetylation of lysine residues at the H3 N-terminus may impact the repair process through multiple means.
Identification of Genes that Function in Parallel with GCN5 to Maintain Genome Stability
Given Gcn5′s recently described roles in DNA replication and repair, we wished to identify other pathways that function in parallel with Gcn5 in the maintenance of genome integrity. Therefore, we set out to identify gcn5Δ double mutants that did not exhibit synthetic growth defects but were sensitive to the DNA damaging agent camptothecin (CPT). The drug CPT results in stabilization of the topoisomerase I-DNA intermediate, ultimately resulting in single-strand and double-strand breaks.Citation63 We performed a yeast genetic screen using the synthetic genetic array (SGA) based strategy,Citation64,Citation65 combining the gcn5Δ mutant with each of ∼4700 yeast deletion mutants. We then challenged the viable double mutants with different concentrations of CPT (). Double mutant cells were scored for growth defects and CPT sensitivity. These mutant cells were verified by individual spot assays where cells of a given genotype were plated in a ten-fold series dilution onto media containing low concentrations of CPT. Finally, we deleted each candidate gene in our laboratory background, W303, and tested how the cells responded to CPT alone or in combination with the gcn5Δ mutant ( and supplemental figure S1). From these tests, we identified ten mutants that were more sensitive to CPT when combined with gcn5Δ than either single mutant alone (). Verifying our screening strategy, one of the genes identified was RTT109, a gene we have shown to function in parallel with GCN5 in the maintenance of genome stability. In addition to CPT, we also determined how each double mutant responded to the DNA damaging agents HU and MMS (, S2 and ). We found that most of the double mutants were sensitive to all DNA damaging agents tested, suggesting that GCN5 and each of these genes are involved in the DNA damage stress response.
The genes that exhibited a synthetic phenotype with GCN5 in response to CPT could be classified into the following groups: checkpoint activation, DNA repair, and other pathways. First, we found that GCN5 genetically interacts with genes involved in checkpoint activation such as RAD9 and RAD24.Citation66 This result is consistent with our published findings that GCN5 genetically interacts with the checkpoint kinases RAD53 and MEC1 and that gcn5Δ mutant cells experience spontaneous chromosome breaks.Citation34 Interestingly, GCN5 only exhibited a synthetic phenotype with RAD9 and RAD24 in response to CPT and HU, but not MMS. Furthermore, the synthetic interaction observed in these double mutants was not as striking as the interaction observed between other genes identified, such as RTT109 ().
Second, we observed that GCN5 genetically interacted with genes implicated directly and indirectly in DNA repair. These genes include MMS4, RPN4, DCC1, and YTA7. Mms4 is a subunit of the Mms4-Mms81 endonuclease involved in DNA damage repair and homologous recombination.Citation67,Citation68 Rpn4 is transcription factor that regulates expression of proteasome genes. In addition, Rpn4 is regulated by various forms of cellular stressCitation69 and localizes to double strand break sites.Citation70 Dcc1 forms a complex with Ctf18 and Ctf8 and is essential for the establishment of sister-chromatid cohesion and DNA replication.Citation71 Sister-chromatid cohesion proteins, including Dcc1, have been found to be important for DNA damage repair.Citation72 Yta7 is predicted to control histone gene expression and occupies similar regions as the histone chaperone Rtt106, Spt4/5/6, and the FACT complex.Citation73 In addition, Yta7 has been identified as one of many proteins in association with chromatin near replication origins.Citation56 It's been suggested that Yta7 functions in an overlapping pathway with Asf1, Hir1 and FACT in response to stress, such as DNA damage.Citation74 This is consistent with our findings that gcn5Δ yta7Δ cells do not show growth defects but do have significant DNA damage sensitivity. It is possible that both Gcn5 and Yta7 control gene expression during times of cellular stress, but it could also suggest that these proteins play a more direct role in DNA damage repair and genome integrity.
Finally, we also observed a significant synthetic interaction between GCN5 and three other genes, MDM20, RPL14A and ELM1. Deletion of each of these three genes, when combined with the gcn5Δ mutant, showed significant sensitivity to all three DNA damaging agents tested (). MDM20 is a subunit of the NatB N-terminal acetyltransferase complex.Citation75 Interestingly, Cac2, one of the components of the CAF-1 complex, is a substrate of NatB. Other potential substrates of the NatB complex include proteins involved in nucleosome assembly, cell cycle progression and DNA processing.Citation76 Rpl14A is a component of the 60S ribosome,Citation77 whereas Elm1 is a serine/threonine kinase with a role in the regulation of cytokinesisCitation78 and determination of cellular morphology.Citation79 While the functions of these three proteins in response to DNA damage stress are unclear, our studies suggest a role for these gene products in the maintenance of genome integrity.
In summary, we found that GCN5 exhibits synthetic interactions with several genes involved in DNA damage response and DNA repair. These genetic interactions provide additional evidence that Gcn5 has a role in the maintenance of genome integrity, and this role may be independent of its role in gene transcription.
Overall Summary
Here, we reviewed recent studies indicating that Gcn5 may have a multitude of functions contributing towards the maintenance of genome stability including roles in DNA replication-coupled nucleosome assembly, initiation of DNA replication, and DNA repair. We also described a screen we performed which identified ten genes that function in parallel to GCN5 in response to DNA damage stress. We hypothesize that one of the primary functions of Gcn5 in DNA replication and repair is the regulation of nucleosome assembly. Gcn5's role in nucleosome assembly may explain many of the phenotypes observed in gcn5Δ cells such as the defects in origin firing and sensitivity to DNA damage agents. Moreover, because cells defective in nucleosome assembly, such as cells lacking CAF-1, are known to affect gene transcription globally,Citation80 it would be interesting to determine to what extent the transcriptional defects observed in gcn5Δ mutant cells are due to compromised nucleosome assembly in this mutant cells. Future studies will shed light on molecular mechanisms by which acetylation and the modifying enzymes including Gcn5 are involved in the processes of DNA replication and repair, two processes contributing to the maintenance of genome integrity.
Figures and Tables
Figure 1 A role for Gcn5 in replication-coupled nucleosome assembly. Rtt109 and Gcn5 are two acetyltransferases that acetylate newly synthesized histone H3. H3 acetylation increases the binding of histones with histone chaperone proteins (CAF-1 and Rtt106) and promotes the deposition of histones onto the newly replicated DNA. Rtt109 predominantly acetylates H3K56, which is important for both CAF-1 and Rtt106 to bind H3. Gcn5 acetylates lysine residues of the H3 N-terminal tail, including H3 lysines 9, 14, 18, 23, and 27, which is important for CAF-1 to bind H3. In addition, Rtt109 is able to acetylate additional residues at the histone H3 N-terminal tail, and this acetylation is a minor contribution compared to Gcn5 (as depicted via a dashed arrow).
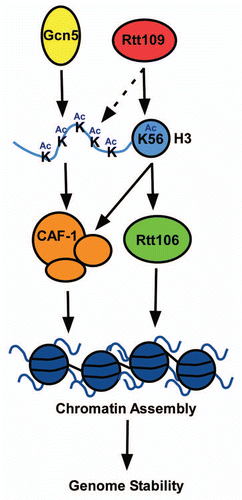
Figure 2 A genetic screen was performed to identify genetic pathways that function in parallel to GCN5 in response to DNA damaging agent CPT. (A) Schematic diagram of the yeast genetic screen for mutants that enhance the sensitivity of gcn5Δ mutant to the DNA damaging agent, camptothecin (CPT). (B) Mutants from the initial screen were evaluated using a spot assay. A ten fold series dilution of yeast cells of the indicated genotype were plated onto regular growth media, YPD, or media containing low concentrations of the DNA damage agents camptothecin (CPT), methyl methanesulfonate (MMS), and hydroxyurea (HU). (C) Summary of the genes found to interact genetically in parallel with GCN5 in response to DNA damage. Those genes in red have roles in checkpoint activation, those in blue have identified roles in DNA repair, and those in green are genes with other functions that act in a parallel manner to GCN5 in response to DNA damage.
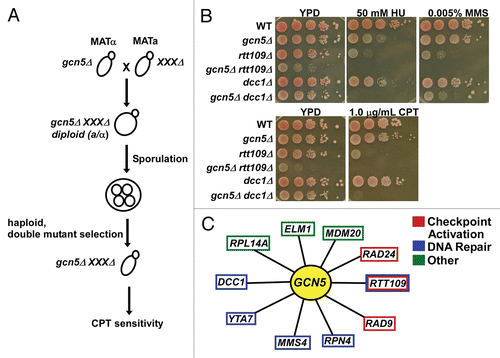
Table 1 Genes that exhibit synthetic genetic interactions with GCN5 in response to DNA damaging agents
Additional material
Download Zip (13.3 MB)Acknowledgements
R.J.B. is supported by an American Heart Association Predoctoral Fellowship and the Mayo Clinic Sydney Luckman Family Predoctoral Fellowship. Z.Z. is a scholar of the Leukemain and Lymphoma Society. Work in the laboratory of Z.Z. is supported by grants from the National Institues of Health.
References
- Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell 2007; 128:721 - 733
- Ransom M, Dennehey BK, Tyler JK. Chaperoning histones during DNA replication and repair. Cell 2010; 140:183 - 195
- Stillman B. Chromatin assembly during SV40 DNA replication in vitro. Cell 1986; 45:555 - 565
- Smith S, Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell 1989; 58:15 - 25
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 2007; 76:75 - 100
- Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature 2001; 411:366 - 374
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57 - 70
- Rocha W, Verreault A. Clothing up DNA for all seasons: Histone chaperones and nucleosome assembly pathways. FEBS Lett 2008; 582:1938 - 1949
- Smerdon MJ. DNA repair and the role of chromatin structure. Curr Opin Cell Biol 1991; 3:422 - 428
- Morrison AJ, Shen X. Chromatin remodelling beyond transcription: the INO80 and SWR1 complexes. Nat Rev Mol Cell Biol 2009; 10:373 - 384
- Gaillard PH, Martini EM, Kaufman PD, Stillman B, Moustacchi E, Almouzni G. Chromatin assembly coupled to DNA repair: a new role for chromatin assembly factor I. Cell 1996; 86:887 - 896
- Moggs JG, Grandi P, Quivy JP, Jonsson ZO, Hubscher U, Becker PB, et al. A CAF-1-PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage. Mol Cell Biol 2000; 20:1206 - 1218
- Kim JA, Haber JE. Chromatin assembly factors Asf1 and CAF-1 have overlapping roles in deactivating the DNA damage checkpoint when DNA repair is complete. Proc Natl Acad Sci USA 2009; 106:1151 - 1156
- Emili A, Schieltz DM, Yates JR 3rd, Hartwell LH. Dynamic interaction of DNA damage checkpoint protein Rad53 with chromatin assembly factor Asf1. Mol Cell 2001; 7:13 - 20
- Jackson V, Shires A, Tanphaichitr N, Chalkley R. Modifications to histones immediately after synthesis. J Mol Biol 1976; 104:471 - 483
- Sobel RE, Cook RG, Perry CA, Annunziato AT, Allis CD. Conservation of deposition-related acetylation sites in newly synthesized histones H3 and H4. Proc Natl Acad Sci U S A 1995; 92:1237 - 1241
- Ai X, Parthun MR. The nuclear Hat1p/Hat2p complex: A molecular link between type B histone acetyltransferases and chromatin assembly. Mol Cell 2004; 14:195 - 205
- Kleff S, Andrulis ED, Anderson CW, Sternglanz R. Identification of a gene encoding a yeast histone H4 acetyltransferase. J Biol Chem 1995; 270:24674 - 24677
- Verreault A, Kaufman PD, Kobayashi R, Stillman B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell 1996; 87:95 - 104
- Tyler JK, Adams CR, Chen SR, Kobayashi R, Kamakaka RT, Kadonaga JT. The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature 1999; 402:555 - 560
- Jasencakova Z, Scharf AN, Ask K, Corpet A, Imhof A, Almouzni G, et al. Replication stress interferes with histone recycling and predeposition marking of new histones. Mol Cell 37:736 - 743
- Masumoto H, Hawke D, Kobayashi R, Verreault A. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature 2005; 436:294 - 298
- Collins SR, Miller KM, Maas NL, Roguev A, Fillingham J, Chu CS, et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature 2007; 446:806 - 810
- Driscoll R, Hudson A, Jackson SP. Yeast Rtt109 promotes genome stability by acetylating histone H3 on lysine 56. Science 2007; 315:649 - 652
- Han J, Zhou H, Horazdovsky B, Zhang K, Xu RM, Zhang Z. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science 2007; 315:653 - 655
- Tsubota T, Berndsen CE, Erkmann JA, Smith CL, Yang L, Freitas MA, et al. Histone H3-K56 acetylation is catalyzed by histone chaperone-dependent complexes. Mol Cell 2007; 25:703 - 712
- Li Q, Zhou H, Wurtele H, Davies B, Horazdovsky B, Verreault A, et al. Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell 2008; 134:244 - 255
- Chen CC, Carson JJ, Feser J, Tamburini B, Zabaronick S, Linger J, et al. Acetylated lysine 56 on histone H3 drives chromatin assembly after repair and signals for the completion of repair. Cell 2008; 134:231 - 243
- Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature 2009; 459:113 - 117
- Yuan J, Pu M, Zhang Z, Lou Z. Histone H3-K56 acetylation is important for genomic stability in mammals. Cell Cycle 2009; 8:1747 - 1753
- Tjeertes JV, Miller KM, Jackson SP. Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. Embo J 2009; 28:1878 - 1889
- Kuo MH, Brownell JE, Sobel RE, Ranalli TA, Cook RG, Edmondson DG, et al. Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature 1996; 383:269 - 272
- Li Q, Fazly AM, Zhou H, Huang S, Zhang Z, Stillman B. The elongator complex interacts with PCNA and modulates transcriptional silencing and sensitivity to DNA damage agents. PLoS Genet 2009; 5:e1000684
- Burgess RJ, Zhou H, Han J, Zhang Z. A role for Gcn5 in replication-coupled nucleosome assembly. Mol Cell 37:469 - 480
- Penn MD, Galgoci B, Greer H. Identification of AAS genes and their regulatory role in general control of amino acid biosynthesis in yeast. Proc Natl Acad Sci USA 1983; 80:2704 - 2708
- Hinnebusch AG, Fink GR. Positive regulation in the general amino acid control of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 1983; 80:5374 - 5378
- Georgakopoulos T, Thireos G. Two distinct yeast transcriptional activators require the function of the GCN5 protein to promote normal levels of transcription. Embo J 1992; 11:4145 - 4152
- Brownell JE, Zhou J, Ranalli T, Kobayashi R, Edmondson DG, Roth SY, et al. Tetrahymena his-tone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 1996; 84:843 - 851
- Kuo MH, Zhou J, Jambeck P, Churchill ME, Allis CD. Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo. Genes Dev 1998; 12:627 - 639
- Grant PA, Duggan L, Cote J, Roberts SM, Brownell JE, Candau R, et al. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev 1997; 11:1640 - 1650
- Pray-Grant MG, Schieltz D, McMahon SJ, Wood JM, Kennedy EL, Cook RG, et al. The novel SLIK histone acetyltransferase complex functions in the yeast retrograde response pathway. Mol Cell Biol 2002; 22:8774 - 8786
- Eberharter A, Sterner DE, Schieltz D, Hassan A, Yates JR 3rd, Berger SL, et al. The ADA complex is a distinct histone acetyltransferase complex in Saccharomyces cerevisiae. Mol Cell Biol 1999; 19:6621 - 6631
- Grant PA, Eberharter A, John S, Cook RG, Turner BM, Workman JL. Expanded lysine acetylation specificity of Gcn5 in native complexes. J Biol Chem 1999; 274:5895 - 5900
- Daniel JA, Grant PA. Multi-tasking on chromatin with the SAGA coactivator complexes. Mutat Res 2007; 618:135 - 148
- Sermwittayawong D, Tan S. SAGA binds TBP via its Spt8 subunit in competition with DNA: implications for TBP recruitment. Embo J 2006; 25:3791 - 3800
- Mohibullah N, Hahn S. Site-specific cross-linking of TBP in vivo and in vitro reveals a direct functional interaction with the SAGA subunit Spt3. Genes Dev 2008; 22:2994 - 3006
- Lee KK, Florens L, Swanson SK, Washburn MP, Workman JL. The deubiquitylation activity of Ubp8 is dependent upon Sgf11 and its association with the SAGA complex. Mol Cell Biol 2005; 25:1173 - 1182
- Ingvarsdottir K, Krogan NJ, Emre NC, Wyce A, Thompson NJ, Emili A, et al. H2B ubiquitin protease Ubp8 and Sgf11 constitute a discrete functional module within the Saccharomyces cerevisiae SAGA complex. Mol Cell Biol 2005; 25:1162 - 1172
- Shukla A, Stanojevic N, Duan Z, Sen P, Bhaumik SR. Ubp8p, a histone deubiquitinase whose association with SAGA is mediated by Sgf11p, differentially regulates lysine 4 methylation of histone H3 in vivo. Mol Cell Biol 2006; 26:3339 - 3352
- Kohler A, Pascual-Garcia P, Llopis A, Zapater M, Posas F, Hurt E, et al. The mRNA export factor Sus1 is involved in Spt/Ada/Gcn5 acetyltransferase-mediated H2B deubiquitinylation through its interaction with Ubp8 and Sgf11. Mol Biol Cell 2006; 17:4228 - 4236
- Zhang W, Bone JR, Edmondson DG, Turner BM, Roth SY. Essential and redundant functions of his-tone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase. Embo J 1998; 17:3155 - 3167
- Lee TI, Causton HC, Holstege FC, Shen WC, Hannett N, Jennings EG, et al. Redundant roles for the TFIID and SAGA complexes in global transcription. Nature 2000; 405:701 - 704
- Fillingham J, Recht J, Silva AC, Suter B, Emili A, Stagljar I, et al. Chaperone control of the activity and specificity of the histone H3 acetyltransferase Rtt109. Mol Cell Biol 2008; 28:4342 - 4353
- Berndsen CE, Tsubota T, Lindner SE, Lee S, Holton JM, Kaufman PD, et al. Molecular functions of the histone acetyltransferase chaperone complex Rtt109-Vps75. Nat Struct Mol Biol 2008; 15:948 - 956
- Espinosa MC, Rehman MA, Chisamore-Robert P, Jeffery D, Yankulov K. GCN5 is a positive regulator of origins of DNA replication in Saccharomyces cerevisiae. PLoS One 5:e8964
- Unnikrishnan A, Gafken PR, Tsukiyama T. Dynamic changes in histone acetylation regulate origins of DNA replication. Nat Struct Mol Biol 17:430 - 437
- Paolinelli R, Mendoza-Maldonado R, Cereseto A, Giacca M. Acetylation by GCN5 regulates CDC6 phosphorylation in the S phase of the cell cycle. Nat Struct Mol Biol 2009; 16:412 - 420
- Tamburini BA, Tyler JK. Localized histone acetylation and deacetylation triggered by the homologous recombination pathway of double-strand DNA repair. Mol Cell Biol 2005; 25:4903 - 4913
- Qin S, Parthun MR. Histone H3 and the histone acetyltransferase Hat1p contribute to DNA double-strand break repair. Mol Cell Biol 2002; 22:8353 - 8365
- Martinez E, Palhan VB, Tjernberg A, Lymar ES, Gamper AM, Kundu TK, et al. Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo. Mol Cell Biol 2001; 21:6782 - 6795
- Brand M, Moggs JG, Oulad-Abdelghani M, Lejeune F, Dilworth FJ, Stevenin J, et al. UV-damaged DNA-binding protein in the TFTC complex links DNA damage recognition to nucleosome acetylation. Embo J 2001; 20:3187 - 196
- Lee HS, Park JH, Kim SJ, Kwon SJ, Kwon J. A cooperative activation loop among SWI/SNF, gamma-H2AX and H3 acetylation for DNA double-strand break repair. Embo J 2010; 29:1434 - 1445
- Liu LF, Desai SD, Li TK, Mao Y, Sun M, Sim SP. Mechanism of action of camptothecin. Ann NY Acad Sci 2000; 922:1 - 910
- Tong AH, Boone C. Synthetic genetic array analysis in Saccharomyces cerevisiae. Methods Mol Biol 2006; 313:171 - 192
- Tong AH, Evangelista M, Parsons AB, Xu H, Bader GD, Page N, et al. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 2001; 294:2364 - 2368
- de la Torre-Ruiz MA, Green CM, Lowndes NF. RAD9 and RAD24 define two additive, interacting branches of the DNA damage checkpoint pathway in budding yeast normally required for Rad53 modification and activation. Embo J 1998; 17:2687 - 2698
- Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ. Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics 2001; 157:103 - 118
- Kaliraman V, Mullen JR, Fricke WM, Bastin-Shanower SA, Brill SJ. Functional overlap between Sgs1-Top3 and the Mms4-Mus81 endonuclease. Genes Dev 2001; 15:2730 - 2740
- Wang X, Xu H, Ju D, Xie Y. Disruption of Rpn4-induced proteasome expression in Saccharomyces cerevisiae reduces cell viability under stressed conditions. Genetics 2008; 180:1945 - 1953
- Ju D, Wang X, Ha SW, Fu J, Xie Y. Inhibition of proteasomal degradation of rpn4 impairs nonhomologous end-joining repair of DNA double-strand breaks. PLoS One 5:e9877
- Mayer ML, Gygi SP, Aebersold R, Hieter P. Identification of RFC(Ctf18p, Ctf8p, Dcc1p): an alternative RFC complex required for sister chromatid cohesion in S. cerevisiae. Mol Cell 2001; 7:959 - 970
- Redon C, Pilch DR, Bonner WM. Genetic analysis of Saccharomyces cerevisiae H2A serine 129 mutant suggests a functional relationship between H2A and the sister-chromatid cohesion partners Csm3-Tof1 for the repair of topoisomerase I-induced DNA damage. Genetics 2006; 172:67 - 76
- Fillingham J, Kainth P, Lambert JP, van Bakel H, Tsui K, Pena-Castillo L, et al. Two-color cell array screen reveals interdependent roles for histone chaperones and a chromatin boundary regulator in histone gene repression. Mol Cell 2009; 35:340 - 351
- Gradolatto A, Rogers RS, Lavender H, Taverna SD, Allis CD, Aitchison JD, et al. Saccharomyces cerevisiae Yta7 regulates histone gene expression. Genetics 2008; 179:291 - 304
- Polevoda B, Cardillo TS, Doyle TC, Bedi GS, Sherman F. Nat3p and Mdm20p are required for function of yeast NatB Nalpha-terminal acetyltransferase and of actin and tropomyosin. J Biol Chem 2003; 278:30686 - 30697
- Caesar R, Warringer J, Blomberg A. Physiological importance and identification of novel targets for the N-terminal acetyltransferase NatB. Eukaryot Cell 2006; 5:368 - 378
- Planta RJ, Mager WH. The list of cytoplasmic ribosomal proteins of Saccharomyces cerevisiae. Yeast 1998; 14:471 - 477
- Bouquin N, Barral Y, Courbeyrette R, Blondel M, Snyder M, Mann C. Regulation of cytokinesis by the Elm1 protein kinase in Saccharomyces cerevisiae. J Cell Sci 2000; 113:1435 - 1445
- Moriya H, Isono K. Analysis of genetic interactions between DHH1, SSD1 and ELM1 indicates their involvement in cellular morphology determination in Saccharomyces cerevisiae. Yeast 1999; 15:481 - 496
- Zabaronick SR, Tyler JK. The histone chaperone anti-silencing function 1 is a global regulator of transcription independent of passage through S phase. Mol Cell Biol 2005; 25:652 - 660