Abstract
Several ETS transcription factors, including MEF/ELF4 and ERG, can function as oncogenes and are overexpressed in human cancer.
ELF4 (also known as myeloid Elf-1 like factor, MEF) belongs to the ETS family of transcription factors, which contains over 30 family members. These have an evolutionarily conserved ETS domain that binds to a consensus “GGAA” DNA sequence via a winged helix-turn-helix motif. While some ETS proteins are transcriptional repressors, most are transcriptional activators (including MEF and ERG). ETS proteins are regulated by mitogenic signaling transduction pathways (e.g., Ras/MAPK), and play important roles in cellular differentiation, proliferation, apoptosis and tissue remodeling. ETS proteins implicated in hematopoietic cell differentiation include PU.1, FLI-1, ETS-1, ETS-2 and TEL. Aberrant expression of ETS proteins have been observed in prostate cancer, breast cancer, sarcoma, glioma and hematological malignancies.
The MEF gene is located on the X chromosome (Xq26) and MEF is normally expressed in many tissues, especially in ovary, placenta, colon and hematopoietic cells.Citation1 MEF activates the expression of a diverse set of target genes (e.g., IL-3, GM-CSF, IL-8, Perforin and MDM2), and also plays a critical role in innate immunity affecting NK cell development and perforin gene expression.Citation2,Citation3
While the MEF gene has been reported to be fused to the ERG gene in a single patient with AML,Citation4 and is overexpressed as result of retroviral insertional mutagenesis in mice,Citation5–Citation7 ERG overexpression is more commonly associated with human cancer. In a remarkable and paradigm changing discovery, a fusion involving the prostate-specific gene transmembrane protease serine 2 gene (TMPRSS2) and the ERG gene was identified in 80% of prostate cancer specimens.Citation8 This gene fusion allows the androgen responsive 5′ regulatory element in TMPRSS2 to control ERG expression, which promotes prostate cancer. TMPRSS2 is also rarely fused to other ETS members such as ETV1, ETV4 and ETV5 in prostate cancer.Citation9 ERG overexpression induces murine epithelial hyperplasia, but it requires collaboration with either PI3K signaling or androgen receptor overexpression to promote the development of prostate cancer in mice.Citation10 The ERG gene is also overexpressed in Ewing's sarcoma (EWS/ERG), peripheral primitive neuro-ectodermal tumors (FUS/ERG), and in AML (also FUS/ERG) via chromosomal translocations. Furthermore, in AML cases with normal cytogenetics, ERG overexpression predicts for a worse outcome than “normal” ERG expression.Citation11 To address the physiological role of ERG in blood cell proliferation, the ERG gene was targeted in mice: a loss of function Erg mutant was shown to impair definitive hematopoiesis and HSC function, demonstrating that Erg is essential for normal hematopoiesis.Citation12 Erg also plays an important role in megakaryocytic differentiation and maturation.Citation13 ERG overexpression promotes the proliferative and self-renewal capacity of HSCs, however overexpression of the FUS/TLS-ERG fusion gene is not sufficient to promote the development of AML.Citation14 These studies imply that ERG overexpression plays a critical role in promoting the growth of various human tumors, however, what ERG target genes contribute to the development of cancer remain largely unknown.
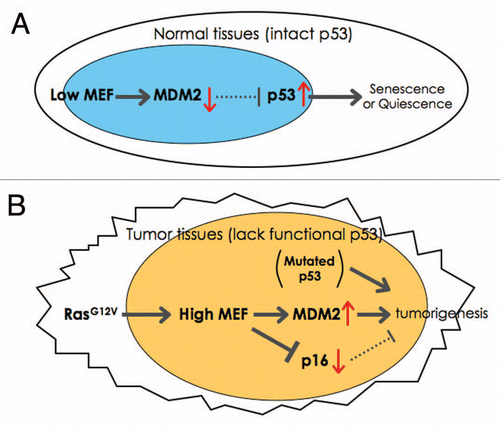
MEF was first isolated approximately 15 years ago. MEF regulates the cell cycle transition from G1 to S phase, thereby promoting cell proliferation. Mef-null mice show greater number of quiescent hematopoietic stem cells (HSCs), indicating that Mef also promotes the transition of HSCs from G0 to G1 phase. By generating Mef-null/p53-null mice we determined that the enhanced quiescence of Mef null HSCs depends on p53 ().Citation15 To further determine the relevance of MEF to human tumors, we examined the level of MEF expression in ovarian cancer and B-cell lymphoma. MEF is overexpressed in ovarian cancer, with protein expression being seen in 48% of serous cystadenocarcinomas, 43% of endometrioid tumors, and 21% of clear cell tumors (where p53 mutations are more common).Citation16 In 26 B-cell lymphoma patient samples with presumably wild type in p53, 23% (6 of 26) showed higher expression of MEF (and enhanced expression of MDM2, see below).Citation17 MEF is also expressed in human AML, with higher expression in poor prognosis subtypes.Citation18 Although the MEF gene is involved in a case of AML with a t(X;21), the main consequence of this translocation, which generates a fusion between MEF and ERG, may be the regulation of ERG expression by the MEF promoter.Citation4 Thus, MEF may contribute to the development of ovarian and hematologic malignancies, consistent with its expression profile in normal human tissues.
To understand the mechanisms by which MEF promotes tumorigenesis, we have recently shown that MEF can cooperate with oncogenic H-RasG12V in promoting transformation.Citation17 In response to oncogenic stress, cells activate both the p53 and p16 tumor suppressor pathways, promoting apoptosis or cellular senescence in order to avoid transformation. In the absence of Mef, mouse embryonic fibroblasts show enhanced senescence with increased p53 expression, due to decreased Mdm2 expression. We determined that this is because Mef binds to and activates the Mdm2 promoter ().Citation17 MEF also has p53-independent effects on tumorigenesis inhibiting Ras/Ets-1 induced p16 expression; thus p53-null/Mef-null mouse embryonic fibroblasts are markedly resistant to H-RasG12V-induced transformation, at least in part due to the accumulated p16 expression (). Thus, MEF promotes the tumorigenesis using both p53-dependent and p53-independent mechanisms.
In earlier studies, we showed that MEF also binds to wild type AML1 (also known as RUNX1) and to the AML1-ETO fusion protein, found in AML with t(8;21). AML1-ETO blocks the transcriptional activating function of MEF,Citation19 and although MEF mRNA is detectable by northern blot in all AML cases studied, AML cases containing AML1-ETO do have lower MEF expression than other subtypes of AML.Citation18 The role of MEF is likely context-dependent;Citation18 however, given that ERG overexpression contributes to the more aggressive behavior of AML with normal cytogenetics, higher levels of Mef could function similarly. Yet, given the effects of MEF on HSC quiescence, the selection of low MEF-expressing cells could occur over time, as Mef-null HSCs are more resistant to chemotherapy or radiotherapy than normal HSCs. At this time, understanding how ETS proteins like MEF and ERG function in tumorigenesis, and how they are regulated by different signaling pathways, remains a priority and a fertile ground for exploration.
Abbreviations
| MEF | = | myeloid Elf1-like factor |
| ELF4 | = | E74-like factor 4 |
| ERG | = | Ets-related gene |
| TMPRSS2 | = | transmembrane protease serine 2 |
| AML | = | acute myeloid leukemia |
| HSC | = | hematopoietic stem cells |
Figures and Tables
Figure 1 A model of how MEF contributes to tumorigenesis. (A) This model shows how low (or null) MEF expression leads to senescence or quiescence due to enhanced p53 expression in normal tissues (e.g., HSCs). (B) The model for transformation in the absence of p53 and the presence of RasG12V (e.g., in ovarian cancer). Oncogenic-Ras can upregulate the expression of MEF. In the absence of wild type p53 (due to genetic deletion or mutations), MEF overexpression promotes transformation by activating MDM2 and suppressing Ras/Ets-1-induced p16 expression.
References
- Miyazaki Y, Sun X, Uchida H, Zhang J, Nimer S. MEF, a novel transcription factor with an Elf-1 like DNA binding domain but distinct transcriptional activating properties. Oncogene 1996; 13:1721 - 1729
- Lacorazza HD, Miyazaki Y, Di Cristofano A, Deblasio A, Hedvat C, Zhang J, et al. The ETS protein MEF plays a critical role in perforin gene expression and the development of natural killer and NK-T cells. Immunity 2002; 17:437 - 449
- Lacorazza HD, Nimer SD. The emerging role of the myeloid Elf-1 like transcription factor in hematopoiesis. Blood Cells Mol Dis 2003; 31:342 - 350
- Moore SD, Offor O, Ferry JA, Amrein PC, Morton CC, Dal Cin P. ELF4 is fused to ERG in a case of acute myeloid leukemia with a t(X;21)(q25-26;q22). Leuk Res 2006; 30:1037 - 1042
- Lund AH, Turner G, Trubetskoy A, Verhoeven E, Wientjens E, Hulsman D, et al. Genome-wide retroviral insertional tagging of genes involved in cancer in Cdkn2a-deficient mice. Nat Genet 2002; 32:160 - 165
- Mikkers H, Allen J, Knipscheer P, Romeijn L, Hart A, Vink E, et al. High-throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nat Genet 2002; 32:153 - 159
- Du Y, Spence SE, Jenkins NA, Copeland NG. Cooperating cancer-gene identification through oncogenic-retrovirus-induced insertional mutagenesis. Blood 2005; 106:2498 - 2505
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005; 310:644 - 648
- Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer 2008; 8:497 - 511
- Zong Y, Xin L, Goldstein AS, Lawson DA, Teitell MA, Witte ON. ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells. Proc Natl Acad Sci USA 2009; 106:12465 - 12470
- Marcucci G, Baldus CD, Ruppert AS, Radmacher MD, Mrozek K, Whitman SP, et al. Overexpression of the ETS-related gene, ERG, predicts a worse outcome in acute myeloid leukemia with normal karyotype: a Cancer and Leukemia Group B study. J Clin Oncol 2005; 23:9234 - 9242
- Loughran SJ, Kruse EA, Hacking DF, de Graaf CA, Hyland CD, Willson TA, et al. The transcription factor Erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. Nat Immunol 2008; 9:810 - 819
- Stankiewicz MJ, Crispino JD. ETS2 and ERG promote megakaryopoiesis and synergize with alterations in GATA-1 to immortalize hematopoietic progenitor cells. Blood 2009; 113:3337 - 3347
- Pereira DS, Dorrell C, Ito CY, Gan OI, Murdoch B, Rao VN, et al. Retroviral transduction of TLS-ERG initiates a leukemogenic program in normal human hematopoietic cells. Proc Natl Acad Sci USA 1998; 95:8239 - 8244
- Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, Huang G, et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 2009; 4:37 - 48
- Yao JJ, Liu Y, Lacorazza HD, Soslow RA, Scandura JM, Nimer SD, et al. Tumor promoting properties of the ETS protein MEF in ovarian cancer. Oncogene 2007; 26:4032 - 4037
- Sashida G, Liu Y, Elf S, Miyata Y, Ohyashiki K, Izumi M, et al. ELF4/MEF activates MDM2 expression and blocks oncogene-induced p16 activation to promote transformation. Mol Cell Biol 2009; 29:3687 - 3699
- Fukushima T, Miyazaki Y, Tsushima H, Tsutsumi C, Taguchi J, Yoshida S, et al. The level of MEF but not ELF-1 correlates with FAB subtype of acute myeloid leukemia and is low in good prognosis cases. Leuk Res 2003; 27:387 - 392
- Mao S, Frank RC, Zhang J, Miyazaki Y, Nimer SD. Functional and physical interactions between AML1 proteins and an ETS protein, MEF: implications for the pathogenesis of t(8;21)-positive leukemias. Mol Cell Biol 1999; 19:3635 - 3644