Abstract
Anaphase-promoting complex/cyclosome (APC/C) is a multifunctional ubiquitin-protein ligase that targets various substrates for proteolysis inside and outside of cell cycle. The activation of APC/C is depended on two WD-40 domain proteins, Cdc20 and Cdh1. While APC/Cdc20 principally regulates mitotic progression, APC/Cdh1 shows a broad spectrum of substrates in and beyond cell cycle. In past several years, numerous biochemical and mouse genetic studies have greatly attracted our attention to the emerging role of APC/Cdh1 in genomic integrity, cellular differentiation and human diseases. This review will aim to summarize the recent expended understanding of APC/Cdh1 in regulating biological function and how its dysfunction may lead to diseases.
Introduction
Ubiquitin-mediated proteolysis regulates various cellular processes, including cell cycle progression.Citation1 Covalent attachment of ubiquitin-chains to target proteins by ubiquitin-protein ligases is a key step to commit these substrates for degradation by the proteasome. SCF (Skp1/CUL1/F-box protein) and APC/C (Anaphase promoting complex/cyclosome) are two well-characterized ubiquitin ligases which govern the sequential and irreversible degradation of dozens of functional regulators inside and beyond cell cycle.Citation2 While several F-box proteins determine substrate-specificity and timing of protein degradation for the SCF complex, two WD40-repeat proteins, Cdc20 and Cdh1, function as substrate adaptors that activate APC/C and specifically recruit substrates for ubiquitylation.Citation2–Citation4 The function of Cdc20 in association with APC/C in triggering the separase-cohesion pathway and mediating spindle-checkpoint signaling during mitosis has been well documented.Citation4,Citation5 The biological function of APC/Cdh1, however, is much broader than that of APC/Cdc20.Citation1,Citation6 Whereas Cdc20 activates APC/C during early mitosis, Cdh1 plays an essential role from late mitosis to the G1/S transitionCitation3 ().
Besides the cell cycle control, recent results in the field have expanded the role of APC/Cdh1 to genomic integrity, signal transduction, cell differentiation and cancer formation.Citation1,Citation6 In the last few years, work from us and others demonstrated that APC/Cdh1 regulates the DNA damage checkpoint response and DNA repair through proteolysis of Rad17, claspin, thymidine kinase 1, TMPK and ribonucleotide reductase R2Citation7–Citation10 ( and B). Furthermore, unscheduled activation of APC/Cdh1 by genotoxic stress has been linked to abrogated G2/M progression, spindle body assembly and chromatid segregationCitation11,Citation12 (). In addition, deregulated Cdh1 has been found to cause aberrant centrosome formation, impaired cytokinesis and DNA re-replicationCitation13 (). Besides its role in genomic integrity, APC/Cdh1 regulates cell differentiation through degradation of cyclin-dependent kinase inhibitors and certain transcriptional inhibitors that are involved in cell cycle withdrawal or onset of terminal differentiationCitation14 (). As a consequence, improper regulation of cell cycle, genomic integrity, signal transduction and differentiation by aberrant Cdh1 activity can contribute to carcinogenesis and other diseasesCitation15,Citation16 ( and ).
Mission for APC/Cdh1 in Mitosis and G1 Progression
Progressive and robust activation of Cyclin B-Cdk1 triggers mitotic entry and promotes APC/Cdc20 activity by phosphorylating APC/C subunits.Citation17 Activated APC/Cdc20 initiates the metaphase-anaphase transition through degradation of cylin B1 and securin.Citation18 To avoid premature separation of sister chromatids, APC/Cdc20 activity is inhibited by Mad2 and BubR1 through the spindle assembly checkpoint (SAC).Citation18 This inhibition is released only when the sister chromatids are aligned at the metaphase plate and bivalent spindle attachments are established.Citation18,Citation19 The decrease of mitotic cyclin-Cdk activity in late mitosis ends the continuous phosphorylation of Cdh1 and thus leads to its partial activation.Citation20 Cdh1 is further activated by release from the inhibition of Acm1, Mad2L2 and Rae1-Nup98.Citation19 Activated Cdh1 replaces Cdc20 in APC/C activation and contributes to the further inactivation of cyclin B-Cdk1 during late mitosis. To achieve this sharp replacement, activation of APC/C by Cdc20 or Cdh1 is oppositely regulated by phosphorylation. Cdc20 can only associate with APC/C when mitotic kinases phosphorylate certain APC/C subunits.Citation17 In contrast, phosphorylation of Cdh1 by Cdk1 or Cdk2 starting from G1/S transition blocks its interaction with APC/C.Citation21 At the end of mitosis and during G1, Cdh1 has to be dephosphorylated to be able to activate APC/C.Citation17,Citation21 In addition, Cdh1 targets Cdc20 for degradation, ensuring the sharp transition from APC/Cdc20 to APC/Cdh1.Citation22 In late mitosis, Cdh1 also mediates the degradation of regulators of cytokinesis and centrosome replication, including Aurora A, Aurora B, Plk1, Anillin and Tpx2Citation13,Citation23,Citation24 ().
In G1, APC/Cdh1 targets several substrates for destruction, including Tome-1, Skp2 and Ets2.Citation25–Citation27 Tome-1 in association with SCF regulates G2/M transition by proteolysis of Wee1. In G1 phase, Tome-1 is removed by Cdh1, thereby allowing accumulation of Wee1 for the next cell cycle progression.Citation27 Skp2 is an F-box protein responsible for the recognition of p21 and p27 by the SCF complex and their subsequent ubiquitylation,Citation28 and Ets2 has been reported to induce cyclin D expression.Citation29 Thus, APC/Cdh1 maintains cells in G1 by accumulating p21 and p27 through Skp2 proteolysis and by limiting the expression of cyclin D1 via promoting Ets2 degradation. Accordingly, depletion of Cdh1 by siRNA stabilizes Skp2 and Ets1, resulting in p21 and p27 degradation and cyclin D1 elevation in G1, followed by premature entry into S phaseCitation25,Citation26 ().
Before S-phase entry, the pre-replication complex (pre-RC) needs to be assembled concomitant with the accumulation of S-phase-specific Cyclin-Cdk activity. To ensure that DNA-replication occurs only once per cell cycle, higher eukaryotes depend on Cdt1 and its inhibitor Geminin.Citation30 Geminin is an APC/Cdh1 substrate during late mitosis, and its degradation releases Cdt1 from inhibition and thus starts the pre-RC assembly.Citation31 During S-phase progression, the accumulation of S-phase-specific cyclin-Cdk activity stabilizes Geminin again through inactivation of APC/Cdh1 during S-phase progression. As a result, Geminin remains in an inhibitory complex with Cdt1 until mitosis, thereby preventing the re-firing of the pre-RC.Citation30 APC/Cdh1 also targets Cdc6, which is essential and limiting for the initiation of eukaryotic DNA replication.Citation32 Considering the importance of inactivation of APC/Cdh1 for cyclin-Cdk accumulation and pre-RC assembly, it is not surprising that overexpression of Cdh1 causes a delay in S-phase onset and reduces the rate of DNA replicationCitation33 ().
To enter S phase, APC/Cdh1 must therefore be inactivated during G1/S transition. Several different mechanisms contribute to APC/Cdh1 inactivation. First, nutrientsstimulated, E2F-dependent transcription leads to accumulation of early mitotic inhibitor-1 (Emi1)/Rca1, which inhibits APC/Cdh1 activity by acting as pseudo-substrate.Citation34,Citation35 Second, this partial inactivation allows the accumulation of cyclin A, which promotes Cdh1 phosphorylation and its dissociation from APC/C.Citation36 Third, phosphorylated Cdh1 itself is targeted for degradation by the SCF ligase.Citation37 Moreover, the degradation of UbcH10 by APC/Cdh1 provides a negative feedback mechanism that would eventually inactivate APC/Cdh1.Citation38,Citation39 Finally, it is reported that Cdh1 can also mediate its own degradationCitation35 ().
The Emerging Role of APC/Cdh1 in Genomic Integrity
To maintain genomic integrity, accurate DNA replication and chromosome segregation are under tight control during cell division. Recent studies implicate Cdh1 as a tumor suppressor essential in maintaining genomic integrity.Citation15,Citation16 There are several lines of evidence in support of this idea. First, Cdh1 is a target tightly regulated by genomic stress and prominently participates in the DNA damage response. Cellular exposure to UV radiation triggers proteolysis of Cdh1, whereas ionizing radiation causes its phosphorylation.Citation40,Citation41 Interestingly, both phosphorylation of Cdh1 and degradation of Cdh1, induced by different DNA-damaging agents, result in a similar effect: the abrogation of cyclin B1 degradation, which is believed to increase Cdk1 activity and enhance apoptosis upon genotoxic stress.Citation41 In support of this, depletion of Cdh1 by gene knockout results in loss of checkpoint function in response to ionizing radiation and enhances the cellular susceptibility to apoptosis, whereas overexpression of non-degradable Cdh1 delays UV radiation-induced apoptosis.Citation41
Another likely consequence of the inactivation of Cdh1, either by phosphorylation or by proteolysis, is to allow the onset of the DNA damage response. Our recent work identified Rad17 as a novel substrate of APC/Cdh1.Citation7 Rad17 was initially identified in fission yeast as a DNA damage checkpoint protein and as an important component of the PCNA-like checkpoint-loading complex.Citation42 The inactivation of Cdh1 after genotoxic stress leads to the accumulation of Rad17 protein. And accumulated Rad17 provides a platform for loading the 9-1-1 checkpointsliding clamp and Claspin after its phosphorylation by ATR.Citation42,Citation43 Recently, Claspin itself has been identified as a novel substrate of APC/Cdh1.Citation8,Citation11 Since the loss of Cdh1 leads to increased Claspin abundance, it is reasonable to assume that elevated Claspin levels will eventually activate the Chk1/p53 pathway to create a DNA damage-like response.Citation8 Following the completion of cellular checkpoint activation after exposure to UV radiation, Rad17 undergoes proteolysis. The destruction of Rad17 results in loss of the platform bridging Claspin to other members of the checkpoint complex.Citation7 The dissociation of Claspin from the checkpoint complexes due to Rad17 proteolysis as well as Claspin degradation disrupts the interaction between ATR and Chk1, a likely mechanism to terminate checkpoint signaling ().
Genotoxic stress leads to cell cycle arrest at G1 or G2 phase to provide sufficient time for cells to repair damaged DNA. Cdh1 plays a role in coordinating the DNA damage response in the cell cycle as its various substrates are active players in both DNA damage response and cell cycle progression ().
Depletion of Cdh1 stabilizes its substrates, such as Cdc6, Prc1 and Geminin, which may contribute to genetic instability by inducing re-replication.Citation31,Citation44 Unscheduled elevation of cyclin A and cyclin B, seen in Cdh1-depleted cells, interferes with loading of the pre-RC.Citation15,Citation16 For instance, Cdh1-depleted mice show reduced Mcm4 and Mcm5 levels on chromatin, suggesting a defect in pre-RC formation in these cells.Citation15 Meanwhile, as complete inhibition of Cdh1 may not be necessary to bypass the G1/S transition, Cdh1 could also participate in regulating origin firing in early S phase.Citation30 As mentioned above, Cdh1 regulates Skp2-mediated p27 and p21 degradation, E2F-dependent cyclin E transcription and Ets2-induced cyclin D expression.Citation25,Citation28,Citation29,Citation45 Cdh1-null cells show a high rate of chromosomal translocations.Citation15,Citation16 This observation can be explained by the premature entry into S phase due to the failure to inactivate the cyclin-Cdk complex in G1 phase. Unscheduled origin firing leads to slow replication from fewer origins. This results in increased stalled replication forks and numerous replication errors, which in turn will activate the p53/p21-dependent replication checkpoint.Citation44 Occasionally, cells will escape the checkpoint and enter mitosis, leading to chromosome breakage or translocation through breakage-fusion-breakage cycles.Citation15,Citation45
Cdh1 is also required for the DNA damage checkpoint in G2.Citation11,Citation46 Upon genotoxic stress in G2, Cdh1 activation by Cdc14B-mediated dephosphorylation leads to degradation of substrates Plk1, Cyclin A and Cyclin B and prevents mitotic entry, opening a time window for DNA damage repair.Citation11 Recent studies also revealed a Cdc14B-independent mechanism of Cdh1 activation: p53/p21-induced reduction of Cdh1 inhibitor Emi1.Citation46 Given that depletion of Cdh1 can activate the p53/p21 pathway in G1 phase, the levels of p21 and Cdh1 seems to be regulated by a positive feedback loop. Previous gene-targeting experiments have shown that disruption of Cdh1 circumvents the G2/M arrest normally induced upon DNA damage. However, mitotic entry is still delayed in Cdh1-null cells, indicating the existence of additional DNA damage checkpoints in G2 phase.Citation11
Genotoxic stress can activate Cdh1 in mitosis too. Under normal circumstances, Cdh1 is inhibited by phosphorylation by Cdk1 and polo kinase.Citation20 DNA damage, however, leads to ATR-mediated inhibition of kinase activity of Cdk1 and polo kinase and thus the activation of Cdh1 in mitosis. Cdh1 targets Cin8/Kip1 and Ase1 to regulate spindle body elongation in early mitosis, while Cdh1-mediated Cyclin B and Cdc20 degradation in anaphase onset prevents the degradation of securin and thus blocks mitotic progression at the metaphase-anaphase transition.Citation12,Citation47 It is noteworthy that securin and Sgo1 are also substrates of Cdh1, suggesting that unscheduled or continuous Cdh1 activation may lead to premature anaphase onset.Citation48,Citation49 This also contributes to the aneuploidy observed in Cdh1-null cells.Citation15
Downregulation of Cdh1, on the other hand, results in the accumulation of its substrates, including Aurora A, Plk1, cyclin A, cyclin B and Cdc20, which are part of the chromosomal instability signature in various murine and human tumors.Citation15,Citation16,Citation50 Their accumulation in murine and human cells leads to genomic instability as evidenced by centrosome aberrations, multipolar mitosis, anaphase bridges and micronuclei.Citation15,Citation16 This is consistent with cultured Cdh1-null MEFs showing substantial numerical and structural chromosomal aberrations.Citation15 Aurora kinases stabilized by Cdh1 inactivation could play a role in observed defects in anaphase spindle organization and premature cytokinesis, which can lead to polyploidy, supernumerary centrosomes and multipolar mitosis.Citation13,Citation15,Citation16 This is consistent with the observed phenotype of Aurora A and Plk1 overexpression.Citation51 Failed degradation of additional substrates, such as Cin8/Eg5 and Kip1 or Ase1/Prc1,Citation12,Citation13 AnillinCitation23 and Tpx2Citation24 may contribute to spindle and cytokinesis defects.
Besides its role in the regulation of the DNA damage checkpoint, APC/C has also been implicated in DNA repair, where APC/Cdh1 targets thymidine kinase 1 (TK1), a key cytosolic enzyme in the salvage pathway for dTTP synthesis, and ribonucleotide reductase R2, a critical enzyme that governs deoxynucleoside triphosphate biogenesis, for degradation. This suggests a key role of Cdh1 in regulating the dNTP pool, important in DNA replication as well as DNA repair.Citation9,Citation10 It is worth noting that an imbalanced dNTP pool may also dramatically increase mistakes in nucleotide incorporation and, consequently, the mutation rate.
APC/Cdh1 Modulates Signal Transduction and Regulates Cellular Differentiation
Considering the importance of Cdh1 in controlling the cell cycle, sensing growth signals and licensing DNA replication, it is not surprising that such a key component was also found to mediate receptor signaling and to regulate cellular differentiation. The following provide examples of such Cdh1-dependent signaling pathways ():
TGFβ.
TGFβ plays both cytostatic and tumor enhancing roles based on the cellular context. In its cytostatic effect, upon stimulation with TGFβ, Smad3 and Smad2 translocate into the nucleus where they interact with both APC/C and SnoN, resulting in the APC/Cdh1-mediated degradation of SnoN that in turn leads to the activation of TGFβ downstream genes and growth inhibition of cultured cells.Citation52,Citation53 Furthermore, our group also demonstrated that TGFβ can trigger APC/Cdh1-dependent Skp2 degradation, which contributes to stabilization of p21 and p27 that is required for tumor suppression.Citation54
NOTCH and JNK.
In Drosophila follicle cells, both Notch signaling and Cdh1 are required for the mitotic-to-endocycle transition.Citation55 Cdh1 is expressed at the mitotic-to-endocycle transition in a Notch-dependent manner and mediates some effects of Notch signaling.Citation55 A nuclear portion of the stress-activated kinase JNK is degraded by APC/Cdh1 during exit from mitosis and G1 phase, suggesting that JNK may be the target of Cdh1-mediated degradation induced by Notch signaling. Expression of a non-degradable JNK induces prometaphase-like arrest and aberrant mitotic spindle dynamics.Citation56
Rb and E2F.
The retinoblastoma protein Rb interacts with APC/Cdh1 and controls the stability of p27 through targeting Skp2 for degradation. By specifically interacting with the hypophosphorylated (active) form of Rb, Cdh1 can regulate the activity of the E2F1 transcription factor.Citation8,Citation33 Meanwhile, E2F stimulates the transcription of early mitotic inhibitor-1 (Emi1)/Rca1, a pseudo-substrate inhibiting APC/Cdh1 activity.Citation35
Ras-MAPK.
It has been well established that Ets2 is activated by the Ras-Raf-MAPK signaling pathway and stimulates cyclin D1 expression, which is the most prominent effect of this important pathway.Citation29 Overactivation of Ras signaling causes oncogene-induced senescence in primary cells through Ets2-mediated increase of p16 expression.Citation57 It is likely that Cdh1 can partially neutralize the overactivation of Ras signaling by degrading Ets2 and thus counteract its effects on p16. Cdh1 could also modulate Ras-induced phenomena, including de-differentiation, tumorigenesis and perhaps even metastatic diseases. Consequently, in normal human fibroblasts, depletion of Cdh1 results in premature senescence, whereas inactivation of both the p53 and Rb pathways by overexpressing SV40 LT-antigen partially reverses this phenotype.
Apoptosis.
The involvement of Cdh1 in apoptotic death was initially reported in B-lymphoma, where overexpression of Cdh1 dramatically increased cell susceptibility to natural killer cell (NK) cytotoxicity.Citation58 A pro-apoptotic effect is also observed in cancer cells treated by UV radiation and in neurons in Alzheimer disease and stroke, sharing a common Cyclin B-Cdk2-triggered apoptotic program.Citation41,Citation59 This notion is also supported by the mitotic catastrophe of Cdh1-depleted tetraploid cells, which is mediated by the apoptotic pathway.Citation51
Glycolysis.
It has recently been reported that Cdh1 targets Pfkfb3, a glycolysis-promoting enzyme, for degradation.Citation60 Pfkfb3 was first identified to be responsible for the different capacities of neurons and astrocytes in tolerating oxidative stress. The accumulation of Pfkfb3 leads to the activation of the glycolysis pathway and promotes cell proliferation, a typical Warburg effect observed in cancer cells.Citation60
Cdh1 directs cellular differentiation mainly at two steps. First, Cdh1 coordinates the balance between cell cycle exit and cell division. Second, Cdh1 promotes differentiation by activating differentiation-licensing factors through degradation of their transcriptional suppressors ().
G1/G0 balance.
Although Cdh1 has previously been suggested to be involved in mitotic exit, results from recent studies have emphasized its critical role in regulating the G1 phase and the quiescent G0 phase. Cdh1 is crucial to keep the mitotic cyclin activity low in early G1 and to regulate the length of G1, leaving time for cell growth and differentiation.Citation1 It has been shown that yCdh1 (FZR1) mutant yeast fail to arrest under nutrient starvation, while dCdh1 (fzr) mutant flies undergo an extra embryonic epidermal cell division.Citation6 Additionally, Cdh1 is not expressed during the early cell divisions of embryogenesis without distinctive G1 or G2 phase, whereas later expression in somatic cells correlates with a long G1 phase.Citation2 The most compelling evidence comes from the inducible deletion of cullin-like Apc2 in mouse liver cells causing quiescent G0 hepatocytes to re-enter the cell cycle without any additional proliferative stimuli.Citation61
In particular, Cdh1 cooperates multiple pathways to maintain the G0/G1 state and to direct cell cycle exit: (1) TGFβ-induced SnoN degradation leads to de-repression of the p15 and p21 promoters; (2) Cdh1 controls Skp2 and Cks1 degradation and consequently the turnover of the CDK inhibitors p27 and p21 by SCFSkp2; (3) Cdh1 inhibits cyclin D transcription by degradation of Ets2; (4) Cdh1 completely eliminates mitotic cyclins, inducing degradation of the CDK1 activator Cdc25A and (5) APC/Cdh1 also directs the degradation of other positive regulators of cell proliferation (for example, Plk1 and Aurora A) and DNA replication (for example, Cdc6, Geminin, Tk1 and Tmpk).
The TGFβ-SnoN-p21/p15 axis is important for the TGFβ-induced neuron growth inhibition, axonal growth, morphogenesis and lens differentiation,Citation62,Citation63 while the Skp2-p27 and Ets2-Cyclin D1 pathways play a key role in myogenesis and differentiation of neuron and neuroblastoma SH-SY5Y.Citation14,Citation64
Moreover, it has been shown that Cdh1 can also regulate cellular senescence and the G1/G0 balance via the APC/Cdh1-Ets2-p16 axis. Indeed, Cdh1-null MEFs proliferate poorly and enter senescence after only a few passages.Citation26
Transcriptional activation of the differentiation licensing factors.
Studies based on cultured mouse myoblasts suggest that myogenesis could be regulated by APC/Cdh1 at different layers. Downregulation of Skp2 by APC/Cdh1 enhances withdrawal from cell cycle through stabilizing p21 and p27, which is necessary for cell differentiation. Moreover, time-dependent destruction of Myf5 was thought to be critical for facilitating myogenic fusion during the process of muscle differentiation.Citation14
Inhibitor of differentiation/DNA binding (Id) proteins modulate cellular proliferation and differentiation in various cell types, such as neural or hematopoietic cells.Citation65 While Id proteins are downregulated during cell cycle exit, overexpression of Id proteins in terminally differentiated cells will trigger cell cycle reentry. It has been reported that Id1, 2 and 4 might be targets of APC/Cdh1 in primary neurons and that Cdh1-dependent Id2 degradation inhibits axon growth and differentiation of neuronal stem cell into neurons.Citation66 Id1 and Id2 also control the proliferation and differentiation in myeloid progenitors. Dysfunction of Id2 in mice or cultured cells caused by deletion or RNA interference induces lymphoid differentiation, whereas Id2 overexpression inhibits lymphoid and myeloid differentiation.Citation65 These Id functions and their regulation by Cdh1 are consistent with the fact that Cdh1+/− mice show plasmacytosis and myelodysplastic syndrome.Citation15
Overall, APC/Cdh1 plays an important role in regulating cellular differentiation. However, present conclusions made based on cell culture systems need to be further validated in animal models.
APC/Cdh1 and Diseases
The multiple functions of APC/Cdh1 discussed above have highlighted its physiological significance in the control of cell cycle, maintenance of genomic stability, transduction of extracellular signaling as well as regulation of cellular differentiation. Combinatorial approaches of biochemistry, cell biology, pathology and mouse genetic models will allow uncovering the relevance of APC/Cdh1 in the pathogenesis of human diseases. As expected, disrupted function of APC/Cdh1 has already been found to lead to various physiological defects that contribute to diseases ().
Carcinogenesis.
Recently xenograft and conditional knockout mouse models have revealed a tumor-suppressing role for APC/Cdh1.Citation15,Citation67 Large-scale human cancer tissue arrays as well as prognostic analyses have further pinpointed the correlation of abrogated APC/Cdh1 function with human carcinogenesis.Citation68,Citation69 Cdh1 heterozygous mice can develop epithelial tumors, plasmacytosis and myelodysplastic syndrome.Citation15 Although these mice are less susceptible to carcinogen-induced skin tumors, by 25 months of age Cdh1 heterozygous mice show a decrease in survival, with 25% of the survivors developing epithelial tumors that are not found in wild-type mice.Citation15 Since only one Cdh1 allele is deleted in these tumors, this suggests that Cdh1 functions as a haploinsufficient tumor suppressor.
Cdh1 downregulation is detected in several cell lines from both hematological neoplasias and solid tumors.Citation16 Cdh1 is also downregulated in many common tumors, including breast, colon, prostate, ovary, liver and brain tumors.Citation11,Citation68,Citation69 Furthermore, deregulation of Cdh1 has also been described in Mantle cell lymphoma and other human non-Hodgkin lymphoma.Citation70
Concomitant with the reduction of Cdh1, several substrates of APC/Cdh1 are frequently overexpressed in cancers with genomic instability, including Cyclin B, Aurora A, Aurora B, Tpx2, Cdc6 and Cdc20.Citation50 A deregulated APC/Cdh1-SCFSkp2-p27 axis has also been reported to play an important role in breast and colorectal cancersCitation68,Citation69 as well as in the malignant progression of a murine B-cell lymphoma cell line.Citation58
Other possible mechanisms of how Cdh1 inactivation may contribute to tumorigenesis have been reported: (1) Cdh1 regulates the susceptibility of cancer cells to the host's natural killer (NK) cell-mediated cell death; however, the underlying mechanism is largely unknown. (2) The glycolysis activation by Pfkfb3 accumulation provides cancer cells tremendous advantages in survival and proliferation.
In summary, the decrease of APC/Cdh1 activity may lead to the accumulation of substrates that may promote the development of highly proliferative, genetically unstable, poorly differentiated and metabolically messed-up cancers capable of escaping our immunosurveillance.
Other diseases.
A role of Cdh1 has also been reported in Alzheimer disease and stroke pathogenesis.Citation59 It has been proposed that Cdk5, triggered either by oxidative stress or Aβ deposition, inactivates Cdh1 by phosphorylation, which allows the accumulation of cyclin B.Citation59 Elevated cyclin B levels will lead to unscheduled activation of Cdk1 and promote apoptotic cell death.Citation41,Citation59
Meanwhile, Cdh1+/− mice show a lot of pathological syndromes such as necrotic liver, liver cysts, plasmacytosis, chronic nephropathy, chronic pancreatitis and myelodysplastic disorders.Citation15 It would be informative to screen the status of Cdh1 and its substrates in tissues of humans affected by analogous diseases of organs such as liver, pancreas and the nervous system. In this regard, crossing Cdh1+/− mice with other disease models will also provide a useful tool. Shedding a new light on Cdh1-mediated diseases hopefully will contribute to the development of putative therapeutic agents for the clinic ().
Future Directions
In the 12 years since the discovery of Cdh1, more and more substrates and regulatory mechanisms have been identified, extending the function of Cdh1 beyond the cell cycle control to genomic integrity, signal transduction, cellular differentiation and carcinogenesis. Nevertheless, little is known about the details of how these components in the APC/C pathway work. Although the rough structure of the APC/C complex has been solved at the electron microscopy level, further studies of the interaction of Cdh1 with its substrates, at both the atomic and molecular level, via crystallography or NMR, are warranted to provide us the structural basis of how Cdh1 recognizes substrates and how Cdh1 mediates substrate ubiquitylation by APC/C.
In addition to substrate recognition, structural studies will also help to reveal how Cdh1 itself is regulated in the various cellular signaling pathways summarized in . For example, previous studies have demonstrated that APC/Cdh1 is tightly regulated during the cell cycle and changes in response to TGFβ signaling as well as genotoxic stress. However, the mechanism by which APC/Cdh1 is regulated is still poorly understood. Studies indicating cell cycle-dependent and phosphorylation-dependent shuttling of Cdh1 between the nucleus and the cytosol will require further dissection using cell biological approaches.Citation71 The recent development of florescence-based systems to monitor cell cycle stages of individual cells will provide useful tools to monitor protein abundance, distribution and even modifications during the cell cycle.Citation51 Regarding modifications, 19 different phosphorylation sites on the Cdh1 homolog in yeast have been identified, most of which are conserved in mammalian cells, allowing a huge combinatorial set of modifications by kinases.Citation72 Such studies may also give new insight on how Cdh1 is regulated by signaling pathways in various cellular processes.
Studies based on biochemistry, mouse genetics and xenograft systems have demonstrated a tumor suppressing role of APC/Cdh1 in various types of cancer. The Cdh1 substrates Aurora A, Aurora B and Plk1 belong to a family of genes associated with tumor formation and most of them are overexpressed in various murine and human cancers. Furthermore, since Cdh1 is also involved in cellular senescence, elucidating how deregulation of APC/Cdh1 enables tumors to overcome the barrier of senescence for malignancy will open a new view for our understanding of the protective mechanisms of APC/Cdh1 in tumorigenesis. Moreover, the development of physiologically relevant chemical inhibitors for APC/Cdh1 will not only enhance studies of APC/C function in the lab but also constitute a rationale anti-cancer therapeutic strategy.
Although Cdh1 has also been linked to neuronal morphogenesis and connectivity, little is known about the underlying mechanism. It will be important to address how APC/C modulates the cytoskeletal dynamics that regulate the morphology and development of the nervous system. Such studies could reveal the role of APC/Cdh1 in learning and memory. Thus, further elucidation of the role of APC/C in brain development, utilizing conditional knockout models, will result in an accurate view for APC/Cdh1 in the pathogenesis of neurological and psychiatric diseases.
Finally, another important still open question regards Cdh1 functions in stem cell biology. We and others have observed the involvement of APC/Cdh1 in embryonic stem cell division, stem cell differentiation and regulation of the hematopoietic cell lineage. However, how APC/Cdh1 regulates the above processes needs to be further addressed.
Figures and Tables
Figure 1 Mission for APC/Cdh1 in mitosis and G1 progression. In mitosis, APC/Cdh1 coordinates with APC/Cdc20 to orchestrate the sequential destruction of mitotic proteins triggering onset of anaphase and further regulating cytokinesis as well as mitotic exit. In coordination with the SCF complex, APC/Cdh1 controls several critical events in G1, including G1/G0 regulation, G1/S phase transition and initiation of DNA replication.
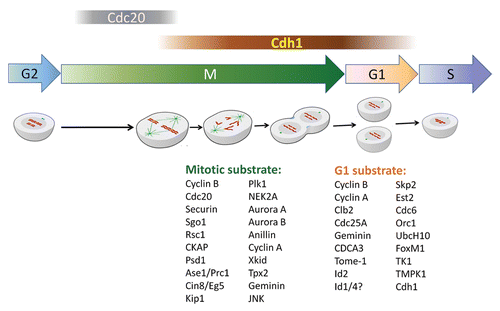
Figure 2 A critical role of APC/Cdh1 in genomic integrity. (A) Emerging functions of Cdh1 in the regulation of the DNA damage checkpoint response. Precise Rad17 proteolysis ensures Rad17 function in both activation and deactivation of the DNA damage checkpoint. In fibroblasts, Rad17 is stabilized following UV to play its role in checkpoint activation. Rad17 destruction by APC/Cdh1 at a later time results in dissociation of claspin, a bridge connecting Chk1 and ATR, from the checkpoint complex. Thereby, degradation of Rad17 after completion of the DNA damage response contributes to termination of checkpoint signaling. (B) Deregulation of APC/Cdh1 may lead to chromosome aberrations and genomic instability. Genotoxic stress leads to unscheduled activation of Cdh1 resulting in abrogated G2/M progression, spindle body assembly and chromatid segregation. Deregulated Cdh1 induces centrosome aberrations and impaired cytokinesis. Moreover, disrupted Cdh1 function leads to genetic instability by inducing DNA rereplication. In addition, APC/Cdh1 is involved in DNA repair through regulating the dNTP pool.
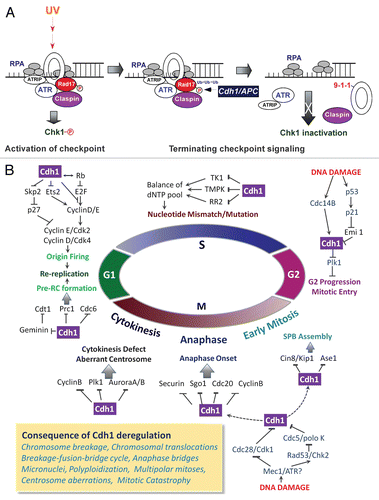
Figure 3 An important role of APC/Cdh1 in coordination between cell division and cellular differentiation. APC/Cdh1 regulates differentiation by controling cell cycle withdrawal and onset of synthesis of certain differentiation related license factors via removal of pertinent transcriptional inhibitors. Stabilization of p27 and p21 via Skp2 degradation by APC/Cdh1, which in turn downregulates CDK2, CDK4 as well as CDK1 activities, is thought to be crucial for cell cycle withdrawal. Destruction of some transcriptional inhibitors such as Id1, 2 and 4 is critical to induce terminal differentiation in certain tissues. Examples include cardiomyocyte generation, myogenesis, neuronal morphogenesis, lens differentiation and bud formation.
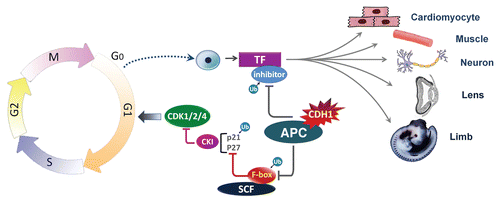
Table 1 APC/Cdh1 substrates and the relevant pathways affected
Table 2 Abrogated protein proteolysis due to deregulated APC/Cdh1 function correlates with various human diseases
Acknowledgements
The laboratory work is kindly supported by NIH grant CA115943. Y. Wan is a scholar of the American Cancer Society.
References
- Li M, Zhang P. The function of APC/CCdh1 in cell cycle and beyond. Cell Div 2009; 4:2
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 2006; 7:2 - 4
- Visintin R, Prinz S, Amon A. CDC20 and CDH1: A family of substrate-specific activators of APC-dependent proteolysis. Science 1997; 278:460 - 463
- Fang G, Yu H, Kirschner MW. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell 1998; 2:163 - 171
- Lorca T, Castro A, Martinez AM, Vigneron S, Morin N, Sigrist S, et al. Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J 1998; 17:3565 - 3575
- Wasch R, Robbins JA, Cross FR. The emerging role of APC/CCdh1 in controlling differentiation, genomic stability and tumor suppression. Oncogene 2010; 29:1 - 10
- Zhang L, Park CH, Wu J, Kim H, Liu W, Fujita T, et al. Proteolysis of Rad17 by Cdh1/APC regulates checkpoint termination and recovery from genotoxic stress. EMBO J 2010; 29:1726 - 1737
- Gao D, Inuzuka H, Korenjak M, Tseng A, Wu T, Wan L, et al. Cdh1 regulates cell cycle through modulating the claspin/Chk1 and the Rb/E2F1 pathways. Mol Biol Cell 2009; 20:3305 - 3316
- Ke PY, Kuo YY, Hu CM, Chang ZF. Control of dTTP pool size by anaphase promoting complex/cyclosome is essential for the maintenance of genetic stability. Genes Dev 2005; 19:1920 - 1933
- Chabes AL, Pfleger CM, Kirschner MW, Thelander L. Mouse ribonucleotide reductase R2 protein: a new target for anaphase-promoting complex-Cdh1-mediated proteolysis. Proc Natl Acad Sci USA 2003; 100:3925 - 3929
- Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 2008; 134:256 - 267
- Juang YL, Huang J, Peters JM, McLaughlin ME, Tai CY, Pellman D. APC-mediated proteolysis of Ase1 and the morphogenesis of the mitotic spindle. Science 1997; 275:1311 - 1314
- Taylor S, Peters JM. Polo and Aurora kinases: Lessons derived from chemical biology. Curr Opin Cell Biol 2008; 20:77 - 84
- Li W, Wu G, Wan Y. The dual effects of Cdh1/APC in myogenesis. FASEB J 2007; 21:3606 - 3617
- Garcia-Higuera I, Manchado E, Dubus P, Canamero M, Mendez J, Moreno S, et al. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat Cell Biol 2008; 10:802 - 811
- Engelbert D, Schnerch D, Baumgarten A, Wasch R. The ubiquitin ligase APC(Cdh1) is required to maintain genome integrity in primary human cells. Oncogene 2008; 27:907 - 917
- Rudner AD, Murray AW. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J Cell Biol 2000; 149:1377 - 1390
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 2007; 8:379 - 393
- Pesin JA, Orr-Weaver TL. Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol 2008; 24:475 - 499
- Crasta K, Lim HH, Giddings TH Jr, Winey M, Surana U. Inactivation of Cdh1 by synergistic action of Cdk1 and polo kinase is necessary for proper assembly of the mitotic spindle. Nat Cell Biol 2008; 10:665 - 675
- Zachariae W, Schwab M, Nasmyth K, Seufert W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science 1998; 282:1721 - 1724
- Pfleger CM, Kirschner MW. The KEN box: An APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev 2000; 14:655 - 665
- Zhao WM, Fang G. Anillin is a substrate of anaphase-promoting complex/cyclosome (APC/C) that controls spatial contractility of myosin during late cytokinesis. J Biol Chem 2005; 280:33516 - 33524
- Stewart S, Fang G. Anaphase-promoting complex/cyclosome controls the stability of TPX2 during mitotic exit. Mol Cell Biol 2005; 25:10516 - 10527
- Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG Jr. Degradation of the SCF component Skp2 in cell cycle phase G1 by the anaphase-promoting complex. Nature 2004; 428:194 - 198
- Li M, Shin YH, Hou L, Huang X, Wei Z, Klann E, et al. The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat Cell Biol 2008; 10:1083 - 1089
- Ayad NG, Rankin S, Murakami M, Jebanathirajah J, Gygi S, Kirschner MW. Tome-1, a trigger of mitotic entry, is degraded during G1 via the APC. Cell 2003; 113:101 - 113
- Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol 1999; 9:661 - 664
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, et al. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem 1995; 270:23589 - 23597
- Diffley JF. Regulation of early events in chromosome replication. Curr Biol 2004; 14:778 - 786
- McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 1998; 93:1043 - 1053
- Petersen BO, Wagener C, Marinoni F, Kramer ER, Melixetian M, Lazzerini Denchi E, et al. Cell cycle- and cell growth-regulated proteolysis of mammalian CDC6 is dependent on APC-CDH1. Genes Dev 2000; 14:2330 - 2343
- Sorensen CS, Lukas C, Kramer ER, Peters JM, Bartek J, Lukas J. Nonperiodic activity of the human anaphase-promoting complex-Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol Cell Biol 2000; 20:7613 - 7623
- Hsu JY, Reimann JD, Sorensen CS, Lukas J, Jackson PK. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nat Cell Biol 2002; 4:358 - 366
- Grosskortenhaus R, Sprenger F. Rca1 inhibits APC-Cdh1(Fzr) and is required to prevent cyclin degradation in G2. Dev Cell 2002; 2:29 - 40
- Lukas C, Sorensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters JM, et al. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature 1999; 401:815 - 818
- Benmaamar R, Pagano M. Involvement of the SCF complex in the control of Cdh1 degradation in S-phase. Cell Cycle 2005; 4:1230 - 1232
- Rape M, Kirschner MW. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature 2004; 432:588 - 595
- Rape M, Reddy SK, Kirschner MW. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell 2006; 124:89 - 103
- Huang X, Tran T, Zhang L, Hatcher R, Zhang P. DNA damage-induced mitotic catastrophe is mediated by the Chk1-dependent mitotic exit DNA damage checkpoint. Proc Natl Acad Sci USA 2005; 102:1065 - 1070
- Liu W, Li W, Fujita T, Yang Q, Wan Y. Proteolysis of CDH1 enhances susceptibility to UV radiation-induced apoptosis. Carcinogenesis 2008; 29:263 - 272
- Wang X, Zou L, Lu T, Bao S, Hurov KE, Hittelman WN, et al. Rad17 phosphorylation is required for claspin recruitment and Chk1 activation in response to replication stress. Mol Cell 2006; 23:331 - 341
- Chini CC, Chen J. Claspin a regulator of Chk1 in DNA replication stress pathway. DNA Repair 2004; 3:1033 - 1037
- Vaziri C, Saxena S, Jeon Y, Lee C, Murata K, Machida Y, et al. A p53-dependent checkpoint pathway prevents rereplication. Mol Cell 2003; 11:997 - 1008
- Ekholm-Reed S, Mendez J, Tedesco D, Zetterberg A, Stillman B, Reed SI. Deregulation of cyclin E in human cells interferes with prereplication complex assembly. J Cell Biol 2004; 165:789 - 800
- Wiebusch L, Hagemeier C. p53- and p21-dependent premature APC/C-Cdh1 activation in G2 is part of the long-term response to genotoxic stress. Oncogene 2010; 29:3477 - 3489
- Jeganathan KB, Malureanu L, van Deursen JM. The Rae1-Nup98 complex prevents aneuploidy by inhibiting securin degradation. Nature 2005; 438:1036 - 1039
- Karamysheva Z, Diaz-Martinez LA, Crow SE, Li B, Yu H. Multiple anaphase-promoting complex/cyclosome degrons mediate the degradation of human Sgo1. J Biol Chem 2009; 284:1772 - 1780
- Jeganathan KB, Baker DJ, van Deursen JM. Securin associates with APCCdh1 in prometaphase but its destruction is delayed by Rae1 and Nup98 until the metaphase/anaphase transition. Cell Cycle 2006; 5:366 - 370
- Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet 2006; 38:1043 - 1048
- Davoli T, Denchi EL, de Lange T. Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell 2010; 141:81 - 93
- Wan Y, Liu X, Kirschner MW. The anaphase-promoting complex mediates TGFbeta signaling by targeting SnoN for destruction. Mol Cell 2001; 8:1027 - 1039
- Stroschein SL, Bonni S, Wrana JL, Luo K. Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes Dev 2001; 15:2822 - 2836
- Liu W, Wu G, Li W, Lobur D, Wan Y. Cdh1-anaphase-promoting complex targets Skp2 for destruction in transforming growth factor beta-induced growth inhibition. Mol Cell Biol 2007; 27:2967 - 2979
- Schaeffer V, Althauser C, Shcherbata HR, Deng WM, Ruohola-Baker H. Notch-dependent Fizzy-related/Hec1/Cdh1 expression is required for the mitotic-to-endocycle transition in Drosophila follicle cells. Curr Biol 2004; 14:630 - 636
- Gutierrez GJ, Tsuji T, Chen M, Jiang W, Ronai ZA. Interplay between Cdh1 and JNK activity during the cell cycle. Nat Cell Biol 2010; 12:686 - 695
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997; 88:593 - 602
- Wang CX, Fisk BC, Wadehra M, Su H, Braun J. Overexpression of murine fizzy-related (fzr) increases natural killer cell-mediated cell death and suppresses tumor growth. Blood 2000; 96:259 - 263
- Maestre C, Delgado-Esteban M, Gomez-Sanchez JC, Bolanos JP, Almeida A. Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity. EMBO J 2008; 27:2736 - 2745
- Bolanos JP, Almeida A, Moncada S. Glycolysis: A bioenergetic or a survival pathway?. Trends Biochem Sci 2010; 35:145 - 149
- Wirth KG, Ricci R, Gimenez-Abian JF, Taghybeeglu S, Kudo NR, Jochum W, et al. Loss of the anaphase-promoting complex in quiescent cells causes unscheduled hepatocyte proliferation. Genes Dev 2004; 18:88 - 98
- Stegmuller J, Huynh MA, Yuan Z, Konishi Y, Bonni A. TGFbeta-Smad2 signaling regulates the Cdh1-APC/SnoN pathway of axonal morphogenesis. J Neurosci 2008; 28:1961 - 1969
- Wu G, Glickstein S, Liu W, Fujita T, Li W, Yang Q, et al. The anaphase-promoting complex coordinates initiation of lens differentiation. Mol Biol Cell 2007; 18:1018 - 1029
- Cuende J, Moreno S, Bolanos JP, Almeida A. Retinoic acid downregulates Rae1 leading to APC(Cdh1) activation and neuroblastoma SH-SY5Y differentiation. Oncogene 2008; 27:3339 - 3344
- Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer 2005; 5:603 - 614
- Lasorella A, Stegmuller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G, et al. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature 2006; 442:471 - 474
- Fujita T, Liu W, Doihara H, Wan Y. An in vivo study of Cdh1/APC in breast cancer formation. Int J Cancer 2009; 125:826 - 836
- Fujita T, Liu W, Doihara H, Date H, Wan Y. Dissection of the APCCdh1-Skp2 cascade in breast cancer. Clin Cancer Res 2008; 14:1966 - 1975
- Fujita T, Liu W, Doihara H, Wan Y. Regulation of Skp2-p27 axis by the Cdh1/anaphase-promoting complex pathway in colorectal tumorigenesis. Am J Pathol 2008; 173:217 - 228
- Lwin T, Hazlehurst LA, Dessureault S, Lai R, Bai W, Sotomayor E, et al. Cell adhesion induces p27Kip1-associated cell cycle arrest through downregulation of the SCFSkp2 ubiquitin ligase pathway in mantle-cell and other non-Hodgkin B-cell lymphomas. Blood 2007; 110:1631 - 1638
- Jaquenoud M, van Drogen F, Peter M. Cell cycle-dependent nuclear export of Cdh1p may contribute to the inactivation of APC/C(Cdh1). EMBO J 2002; 21:6515 - 6526
- Hall MC, Warren EN, Borchers CH. Multi-kinase phosphorylation of the APC/C activator Cdh1 revealed by mass spectrometry. Cell Cycle 2004; 3:1278 - 1284
- Gao D, Inuzuka H, Tseng A, Wei W. Akt finds its new path to regulate cell cycle through modulating Skp2 activity and its destruction by APC/Cdh1. Cell Div 2009; 4:11
- Kitajima S, Kudo Y, Ogawa I, Tatsuka M, Kawai H, Pagano M, et al. Constitutive phosphorylation of aurora-a on ser51 induces its stabilization and consequent overexpression in cancer. PLoS One 2007; 2:e944
- Pan HW, Chou HY, Liu SH, Peng SY, Liu CL, Hsu HC. Role of L2DTL, cell cycle-regulated nuclear and centrosome protein, in aggressive hepatocellular carcinoma. Cell Cycle 2006; 5:2676 - 2687