Neural progenitor cells are multipotent cells which retain the ability to self-renew and differentiate into neurons, astrocytes and oligodendrocytes in culture.Citation1 Neural stem cells have been identified not only in the developing brain but also in the adult nervous system of mammals, including humans.Citation2 Within the adult central nervous system two areas, the subventricular zone (SVZ) and the hippocampal subgranular zone, are characterized by the presence of dividing cells that possess the ability to differentiate into neural lineages. When exposed to a high concentration of mitogens such as fibroblast growth factor-2 (FGF2) or epidermal growth factor (EGF), the primary cells extracted from those areas of the brain are promoted to proliferate in culture preserving the undifferentiated state.Citation3,Citation4
Fibroblast growth factor-2 has pleiotropic effects on the embryonic and adult nervous systemCitation5,Citation6 and recently our group has identified a novel regulatory pathway orchestrated by FGF2 and resulting in the upregulation of the co-chaperone BAG3 in SK-N-MC neuroblastoma cell line.Citation7 BAG3 is an 80 KDa protein characterized by interaction with a variety of partners such as Hsp-70 and Bcl-2 and its expression has been linked to the regulation of cellular events including cell survival and apoptosis.Citation8 Earlier studies have demonstrated that brain injuries such as ischemia and kainic-acid induced seizures correlate with an induced expression of BAG3 in the damaged area.Citation9,Citation10 Nevertheless, the role of BAG3 in the central nervous system has not yet been elucidated, particularly in terms of proliferation and survival.
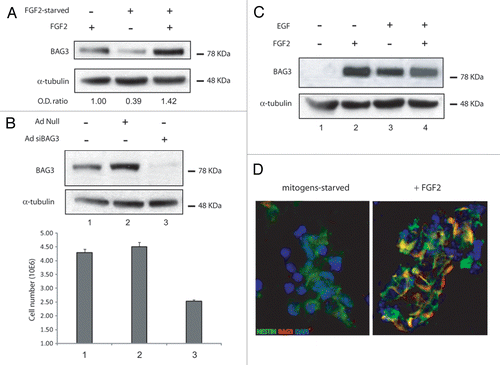
These speculations prompted us to examine the level of BAG3 expression in adult rat hippocampal neural progenitors. As shown in (lane 1), passage 25 neural progenitor cells, isolated from hippocampus and characterized elsewhere,Citation1 expressed BAG3 protein at moderate levels when cultured in Neurobasal medium supplemented with N2 plus FGF2. When cells were subjected to FGF2-starvation, BAG3 expression dropped by 60% (lane 2). The addition of FGF2 to culture media devoid of the growth factor restored the basal level of BAG3 (lane 3), suggesting that FGF2 sustains BAG3 expression in neural progenitor cells. This data is in accordance with earlier observations on SK-N-MC neuroblastoma cells.Citation7 The overnight FGF2 starvation, though, did not change the neural progenitor features of the cells, as verified by qPCR analysis of the markers of stemness (SOX2 and Nestin) and neural lineages (type III β-tubulin, GFAP, O4) (data not shown).
As FGF2 plays an important role in the proliferation of neural progenitors, we sought to investigate the impact of FGF2-driven BAG3 expression on the growth rate of neural stem cells. To this end cells were treated with an adenovirus expressing siRNAs targeting the BAG3 mRNA and three days after infection protein analysis and cell number were measured. As shown in , treatment of neural progenitor cells with Ad-siBAG3, but not Ad-null, suppressed BAG3 expression in the presence of FGF2. Accordingly, reduced level of BAG3 determined a decrease in the cell number greater than 45%, indicating that BAG3 does have a role in the proliferation rate of neural progenitor cells.
In the next series of experiments we investigated the potential of the mitogens FGF2 and EGF to stimulate BAG3 expression in secondary neurospheres extracted from the developing brain of mouse embryo. As described previuosly,Citation11 primary neurospheres are non-adherent spherical structrures mainly constituted of nestin-positive cells (neural stem cells) which maintain their primitive ontogenetic state when cultured in presence of mitogens such as FGF2 and EGF, and in the absence of substrate attachment.
Secondary neurospheres were obtained as described in the Figure legend. After a 10 h starvation from growth factors, the cells were readministrated with FGF2 or EGF or FGF2/EGF added together. As shown in , the level of 80 KDa BAG3 protein was undetectable when secondary neurospheres were starved from mitogens (lane 1). 48 h after readministration, BAG3 expression was noticeably induced at comparable levels either in FGF2- (lane 2), EGF- (lane 3) or EGF/FGF2-treated cells (lane 4).
To confirm that the stimulatory effect of FGF2 on BAG3 expression occurred in nestin-positive cells, secondary neurospheres starved of growth factors (left part) or readministrated with FGF2 (right part) were double labeled with BAG3 antibody and the progenitor marker nestin antibody (). According to western blot data, mitogen-starved neural progenitors display undetectable BAG3 levels in nestin positive cells, while Rhodamine-labeled BAG3 antibody colacalizes with FITC-labeled nestin antibody in FGF2-treated cells. These observations confirm that BAG3 induction upon FGF2 treatment is also detected in cultures of neural progenitor cells at early passage number.
Our data support the evidence that FGF2 sustains BAG3 expression in rat neural progenitor cells, as indicated by decrease in BAG3 levels upon FGF-2 starvation. In SK-N-MC neuroblastoma cells FGF2-driven BAG3 upregulation impacts on the G2/M cell cycle phase transition.Citation7 Accordingly, targeted suppression of BAG3 by siRNA reduced neural progenitor cell number (). Nevertheless, the mechanism underlying this event still remains to be elucidated.
The BAG3 level in the brain has a low expression profile. Moreover there are no major phenotypical alterations described in the BAG3-KO mouse brain.Citation12 In light of these considerations, BAG3 does not seem to be essential for the CNS development. In adulthood, though, stem cells play an essential homeostatic role in replacing differentiated cells that are lost following physiological turnover, injury or disease. Brain injuries such as ischemia and seizures correlate with BAG3 overexpression in the damaged area.Citation9,Citation10 From this point of view BAG3 seem to have a more relevant role in response to stress in adulthood.
Figures and Tables
Figure 1 (A) FGF2 sustains BAG3 protein levels and impacts the proliferation of neural progenitor cells. Western blot analysis of total lysates from adult hippocampus-derived neural progenitor cells (passage number 25) cultured in neurobasal medium (NM) containing N2 supplement plus 20 ng/ml fibroblast growth factor-2 (lane 1), FGF-2-starved cells (lane 2), cells starved and then readministrated with FGF-2 (lane 3). BAG3 endogenous protein was evaluated by imunoblotting with a rabbit polyclonal antibody against BAG3. α-tubulin was detected as normalization control among samples. (B) Adenoviral infection of BAG3 siRNA was performed where indicated at 40 m.o.i. per cell. The cells were FGF2-starved 10 h and then treated with FGF2 for 72 h. Cells were counted and cell lysates were evaluated for BAG3 protein expression. The data reported in (B) are the average of 3 independent experiments. Percentage of viability by trypan blue exclusion did not show significant change among different samples (data not shown). (C) BAG3 expression is induced by EGF and FGF-2 in secondary neurosphere and localizes in nestin posive cells. FGF2 and EGF sustain BAG3 protein expression. Primary neurospheres were prepared from E16 mouse embryos and cultured in neurobasal medium containing N2 supplement plus FGF2 20 ng/ml and EGF 20 ng/ml. After splitting, secondary neurospheres have been cultured for 10 h in N2 supplement (lane 1) or for additional 48 h in N2 + FGF2 (lane 2) or N2 + EGF (lane 3) or N2 + FGF2/EGF (lane 4). (D) Mouse secondary neurospheres where cultured as indicated in (C). Imunofluorescence performed with FITC-labeled anti-nestin antibody and rhodamine-labeled BAG3 antibody are shown in neurobasal medium + N2 cultured cells (left) and neurobasal + N2 + FGF2 20 ng/ml cultured cells (right).
Acknowledgements
The authors wish to thank past and present members of the Department of Neuroscience and Center for Neurovirology for sharing of ideas and reagents, helpful advice and continued support. In particular we thank Drs. Francesca Peruzzi, Krzysztof Reiss and Giovanni Passiatore and Ms. Elisa Gualco for their advice and assistance. We also thank C. Schriver for editorial assistance. This work was made possible by grants awarded by NIH to K.K.
References
- Hsieh J, et al. J Cell Biol 2004; 164:111 - 122
- Gage F. Science 2000; 287:1433 - 1438
- Gensburger C, et al. FEBS Lett 1987; 217:1 - 5
- Reynolds B, et al. Science 1992; 255:1707 - 1710
- Temple S, et al. Neuron 1995; 15:249 - 252
- Gritti A, et al. J Neurosci 1996; 16:1091 - 1100
- Gentilella A, et al. Oncogene 2008; 27:5011 - 5018
- Rosati A, et al. Int J Biochem Cell Biol 2007; 39:1337 - 1342
- Lee M, et al. Exp Mol Med 2002; 34:167 - 171
- Lee M, et al. Exp Neurol 2002; 175:338 - 346
- Marshall Gn, et al. Methods Mol Biol 2008; 438:135 - 150
- Homma S, et al. Am J Pathol 2006; 169:761 - 773