Abstract
Extracellular matrix (ECM) is a key regulator of tissue morphogenesis and functional differentiation in the mammary gland. We showed recently that laminin-111 (LN1) together with prolactin induces b-casein expression in mammary epithelial cells (MECs) by sustaining STAT5 activation. Others have shown that Rac1 is required for integrin-mediated STAT5 activation, but molecules upstream of Rac1 remain to be elucidated. Here, we show that exposure to three-dimensional (3D) laminin-rich ECM (LrECM) gels changes the localization of phosphoinositide 3-kinase (PI3K) in MECs from diffuse to basal accompanied with the activation of PI3K-Rac1 signaling pathway. We show by co-immunoprecipitation that Rac1 associates with STAT5, and that LrECM treatment enhances this interaction. Blocking PI3K with LY294002 inhibits LrECM-dependent Rac1 activation, attenuates sustained STAT5 phosphorylation, and blocks ß-casein gene transcription. These results indicate that PI3K is a key mediator of the LN1-induced signaling cascade which controls the activity of transcription factors essential for tissue-specific gene expression.
Introduction
The family of signal transducers and activators of transcription (STATs) consists of seven structurally homologous proteins that play important and distinct roles in the regulation of organ development and cell differentiation.Citation1 In the cytoplasm, latent STATs are activated by hormone-, cytokine- and growth factor-stimulated tyrosine phosphorylation. STATs dimerize after phosphorylation and translocate into the nucleus where they bind to and modulate the transcription of genes containing gamma interferon activation sequences (reviewed in ref. Citation1 and Citation2). STAT5 is an important component of the prolactin signaling pathway in MECs and regulates β-casein expression in culture and in vivo.Citation3,Citation4 The canonical prolactin receptor (PrlR)-STAT5 signaling pathway is initiated by prolactin binding and transduced via JAK2-induced STAT5 phosphorylation.Citation5,Citation6 Deletion of PrlR, JAK2 or STAT5 impairs alveologenesis and lactation in MECs, suggesting that the PrlR-STAT5 signaling pathway is essential for mammary gland development and function.Citation7–Citation9
MECs isolated from the mammary gland and grown on tissue culture plastic (2D cultures) fail to respond to prolactin and are unable to activate STAT5 and subsequent mammary-specific gene expression.Citation10,Citation11 We have shown that the responsiveness of MECs to prolactin is dependent on correct exposure of PrlR to circulating hormone, which is at the basal side of acini in vivo. In monolayer, MECs have a limited capacity for binding to apically-placed prolactin because the PrlR is distributed along the basolateral surface. Culturing MECs as aggregates on non-adhesive substrata exposes the PrlR, allowing it to bind to prolactin and activate STAT5 in the absence of LN1.Citation12 This activation, however, is only transient and not sufficient to stimulate transcription of mammary-specific genes.Citation12 When treated with LN1 or LrECM, MECs reorganize into polarized acini allowing the exposure of PrlR to prolactin. But in this case, exposure to prolactin leads to sustained STAT5 phosphorylation and high levels of STAT5 nuclear translocation.Citation12 We hypothesized that the binding of LN1 to its receptors activates specific biochemical pathways that prolong STAT5 activation.
MECs bind to laminin through β1-integrin subunit and dystroglycan (DG).Citation13,Citation14 Both receptors play important roles in mammary gland function by promoting nuclear translocation and/or the sustained activation of STAT5.Citation14,Citation15 In the present study, we show that it is LN1 reorganizing non-polar MEC aggregates into polarized structures, which allows preferential expression of PI3K on the basal surface of the acini. Accompanying these structural changes is the activation of the PI3K-Rac1 pathway, an event that is necessary for sustained STAT5 activation and mammary-specific gene expression. We also found that STAT5 binds to Rac1 and that this interaction is enhanced by LrECM. These results suggest that the PI3K-Rac1 pathway most probably allows integration of the ECM and lactogenic hormone signals to induce and maintain STAT5 activation, an essential event for MEC functional differentiation.
Results
Sustained STAT 5 activation correlates with cell polarization.
We showed that LN1 cooperates with prolactin to sustain STAT5 activation and induces acinar morphogenesis in MECs.Citation12 We asked whether there is a relation between the onset of mammary epithelial acinar morphogenesis and sustained STAT5 activation. EpH4 cells were cultured on nonadhesive, polyHEMA-coated dishes for 24 hours and treated for different time intervals with prolactin in the presence or absence of LrECM, a cost-effective surrogate for LN1. Cell polarization was assessed by immunofluorescence (IF) staining with antibodies against α6-integrin (basal marker) and ZO-1 (apical marker). In the absence of LrECM, EpH4 cells assembled into non-polarized spheroid structures, which displayed lateral staining for α6-integrin and ZO-1 (). In contrast, after 24 hours of LrECM treatment, α6-integrin and ZO-1 were labeled on the basal and apical surfaces, respectively, suggesting that the cells formed polarized acinus-like structures. Western blot analysis showed that STAT5 phosphorylation was induced transiently and irrespective of LrECM treatment after addition of prolactin for 15 minutes. However, prolonged incubation with LrECM led to sustained reactivation of STAT5 at around 24 hours (), a time that correlated with the temporal effect of LN1 on cell polarity.
We extended these findings by determining whether the effects of LN1 on polarity and sustained STAT5 coincided also with transcriptional activation of the β-casein gene, a downstream transcriptional target of activated STAT5. To accomplish this, we produced a β-casein promoter-CFP reporter construct (), stably expressed it in EpH4 cells cultured on a non-adhesive poly-HEMA surface and monitored the CFP levels by fluorescence microscopy. We found that activation of the β-casein promoter required both LN1 and prolactin signals and that inhibition of sustained STAT5 activation with the JAK2 inhibitor AG490 dramatically blocked this activation (). These results indicate that LN1-dependent sustained STAT5 activation is necessary for transcriptional activation of the β-casein promoter and therefore functional differentiation in MECs.
PI3K is required for sustained STAT 5 activation.
The effect of LN1 on MEC morphogenesis and functional differentiation is mediated, in part, through cell surface integrin receptors.Citation15,Citation16,Citation17 PI3K is an important mediator of integrin signaling to regulate cellular architecture and proliferationCitation18,Citation19 and is basally localized in polarized MECs.Citation18 Using immunofluorescence analysis, we observed that PI3K was diffusely distributed throughout the cytoplasm of cells cultured on poly-HEMA and that exposure to LrECM for 24 hours caused PI3K to re-localize to the basal surface (indicated by labeling with the basal marker α6-integrin in ).
The profound effect of LN1 on PI3K cellular localization led us to test the effect of this ECM molecule on the activity of the small GTPase Rac1, a downstream target of PI3K. Using GST pull-down assays for GTP-Rac1, we showed that LrECM treatment enhanced the Rac1 activity by about two-fold ().
Rac1 has been shown to interact with and to stimulate the phosphorylation of STAT3 in COS-1 cells.Citation20 Therefore, we asked whether Rac1 binds to STAT5 directly in MECs and whether this binding is regulated by LN1. Rac1-associated proteins were precipitated with an antibody against Rac1 from EpH4 cells that were cultured on polyHEMA in the presence or absence of LrECM. Western blot analysis of the immunoprecipitate showed a direct interaction between Rac1 and STAT5 and a three-fold enhancement of this interaction in response to LrECM treatment ().
These findings suggest a role for the PI3K pathway in mediating LN1-induced sustained STAT5 activation. To conclusively demonstrate such a role, we treated functionally differentiated EpH4 cells with LY294002 (LY), a small molecule PI3K inhibitor. This treatment inhibited sustained STAT5 phosphorylation (), down-modulated LN1-induced Rac1 activation () and attenuated LrECM-enhanced interaction of STAT5 with Rac1 (). The same treatment also drastically reduced both β-casein RNA levels () and promoter activity (). These data indicate that the PI3K-Rac1 pathway is required for sustained LrECM-induced STAT5 activation and, therefore, essential for mammary-specific gene expression.
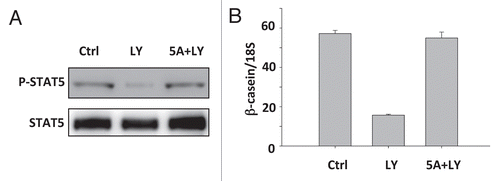
We showed previously that expression of a constitutively activated STAT5 (containing two amino acid substitutions, H299R and S711F) prolonged tyrosine phosphorylation and nuclear translocation and induced β-casein expression in mammary epithelial cells in the absence of LrECM.Citation12 Given that inhibition of PI3K blocks sustained STAT5 phosphorylation and mammaryspecific gene expression, we wondered whether a constitutively activated STAT5 would rescue the mammary-specific function in LY-treated cells. EpH4 cells were infected with a retrovirus expressing the constitutively activated STAT5A. Infected cells were cultured on polyHEMA-coated dishes and then treated with Prl and LrECM for 8 hours before adding LY. Expressing constitutively active STAT5 rescued LY-mediated inhibition of sustained STAT5 phosphorylation () and β-casein transcription (). Therefore, STAT5 is a major target through which the PI3K-Rac1 pathway regulates functional differentiation in MECs.
Discussion
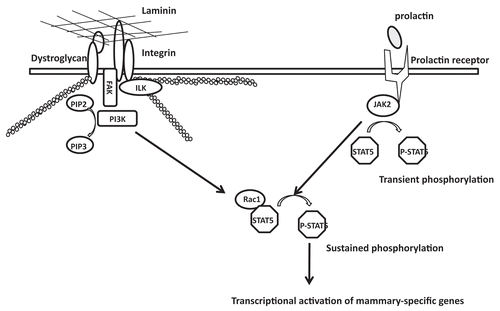
The dynamically reciprocal dialogue of the ECM and the nucleus is crucial for transcriptional regulation; this in turn dictates tissue morphogenesis and function.Citation21,Citation22 The identification of an ECM-regulated transcriptional element in the promoter of a casein gene suggests the existence of ECM-regulated transcription factors and provides a clue for how the ECM may coordinate gene expression and functional differentiation.Citation23,Citation24 Indeed, STAT5 is modulated by the ECM to regulate tissue-specific gene expression.Citation10,Citation11,Citation25,Citation26 In addition, it has been shown that LN1 establishes polarity, induces quiescence and promotes the relocalization of several nuclear proteins in mammary epithelial cells, such as NuMA, the RNA splicing factor SRm160, NFκB, TIN2 and the cell cycle regulator, Rb.Citation27,Citation28 However, the signaling pathways through which LN1 communicates with the nucleus to influence nuclear organization and mammary-specific gene expression remained, for the most part, undiscovered. Here, we show that PI3K localization and activity are regulated by LN1 signaling in MECs, and that activation of PI3K is required for sustained STAT5 phosphorylation and milk protein expression. Thus, the PI3K-Rac1 pathway represents a LN1-induced signaling network that functions through STAT5 to transmit signals from the ECM to the nucleus, which, in turn, regulate mammary gland function ().
For a long time now, we have known that mammary-specific gene expression requires signals from integrins and lactogenic hormone receptors.Citation30 Deletion of β1-integrin impairs STAT5 phosphorylation and nuclear translocation in epithelial cells during lactation.Citation15 It has been proposed that dystroglycan, which also binds LN1, assists in assembling LN1 into a basement membrane,Citation14 and that this event enhances STAT5 activation by promoting further canonical signaling through integrins. In support of this, we recently showed that dystroglycan is required for sustained STAT5 activation and mammary-specific gene expression.Citation12 However, deletion of the cytoplasmic domain of DG has little effect on STAT5 activation and milk protein expression, suggesting that the binding of LN1 to DG does not appear to induce the biochemical signals directly. Thus, β1-integrin is likely the main ECM receptor that induces the biochemical signals necessary for sustained STAT5 activation.
We have shown previously that Rac1, a downstream target of PI3K, regulates tissue polarity in 3D cultures of human breast epithelial cells, and that PI3K and PIP3 are localized at the basal surface of the polarized acini.Citation18 Here we observed also the basal localization of PI3K in non-malignant mouse mammary epithelial cells, and, additionally, we showed the concomitant activation of Rac1 in response to LrECM treatment. Blocking PI3K inhibits both Rac1 activation and ECM-induced STAT5 activation and, correspondingly, reduces mammary-specific gene expression. The introduction of dominant-negative Rac1 blocks STAT5 activation and β-casein expression in 3D LrECM gels,Citation31 suggesting that the PI3K-Rac1 pathway is crucial for mammaryspecific function. Rac1 has been shown to interact with STAT5A and enhance its nuclear translocation as a chaperone in hematopoietic cells.Citation32 We show further that Rac1 binds to STAT5 in MECs, and that this binding is enhanced in the presence of LN1. Since LN1 treatment has little effect on JAK2 activity (data not shown), it is possible that Rac1 recruits STAT5 to the kinase complexes to prolong its phosphorylation.
Whereas the evidence provided here describes a clear role for PI3K in mammary-specific gene expression, the factors that signal upstream of this molecule remain to be discovered. A recent study showed that conditional deletion of integrin-linked kinase (ILK) in MECs inhibits the phosphorylation and nuclear translocation of STAT5 as well as milk protein expression.Citation33 Mammary-specific deletion of focal adhesion kinase (FAK) inhibits phosphorylation of STAT5 and significantly reduces expression of whey acidic protein in the mammary gland.Citation34 Since PI3K has been identified as a downstream target of ILK and FAK,Citation35,Citation36 it is possible that these two molecules mediate signal transduction from integrin to PI3K ().
In summary, we have identified PI3K-Rac1 as the signaling pathway through which LN1 sustains activation of STAT5, allowing for mammary-specific gene expression. During breast cancer development, the PI3K-Rac1 pathway is dysregulated and STAT5 is activated,Citation18,Citation37,Citation38 underscoring the validity of our current findings for potentially providing insight into mechanisms responsible for cancer development.
Material and Methods
Antibodies and reagents.
The following antibodies and culture reagents were obtained as indicated: STAT5 and Lamin A/C (Santa Cruz Biotechnology); phosphorylated STAT5 and Rac1 pull-down kit (Upstate Biotechnology); α6-integrin (Chemicon); ZO-1 (a kind gift from Dr. Masahiko Itoh, Dokkyo University Tochigi prefecture, Japan); PI3K (Transduction Laboratory); LN1 (Trevigen); Matrigel® (BD Biosciences); LY294002 (Cell Signaling); protease inhibitor cocktail 1 (CalBiochem).
Cell lines and constructs.
EpH4 cells were originally isolated from a mid-pregnant mouse and became immortalized in culture.Citation39 Cells were maintained in DMEM/F12 (UCSF Cell Culture Facility) supplemented with 2% fetal bovine serum (GIBCO-BRL), 50 µg/ml gentamycin and 5 µg/ml insulin (Sigma). To make nonadhesive substrata, polyHEMA was dissolved in 95% ethanol at 6 mg/ml and plates were coated at 0.25 mg/cm2 as previously described.Citation40 EpH4 cells were cultured on polyHEMA-coated plates at 30,000 cells/cm2. 24–48 hours after plating, cells were collected by centrifugation and resuspended in DMEM/F12 medium containing insulin, hydrocortisone and relevant ECM components.
The β-casein promoter was amplified from mouse genomic DNA using the following primers: 5′-TTC ATA ACT GAG GTT AAA GCC-3′; reverse primer, 5′-GTC CTA TCA GAC TCT GTG AC-3′. The amplified promoter fragment was cloned into pECFP-C1 (Clontech) using the strategy described in . The resulting vector was transfected into EpH4 cells and selected with G418. G418-resistant clones were cultured on top of LrECM gel and treated for 48 h with prolactin and hydrocortisone to induce β-casein promoter actiation. A clone displaying the strongest CFP signal (indicative of promoter activity) in response to lactogenic hormones was identified, expanded and used in this study. A construct containing STAT5A 1*6 (a kind gift from Dr. Toshio Kitamura, University of Tokyo, Japan) was introduced by transfection into Phoenix packaging cells, and retroviral stocks were produced according to standard protocols. MECs were infected at 40–50% confluence.
Co-immunoprecipitation and western blot.
Cells grown on plastic were lysed in situ in RIPA buffer [1% Nonidet P-40, 0.5% deoxycholate, 0.2% SDS, 150 mM sodium chloride, 50 mM Tris·HCl (pH 7.4) containing phosphatase and protease inhibitor cocktails (Calbiochem)]. Cells on polyHEMA were collected by centrifugation and lysed in RIPA buffer. Equal amounts of protein lysates were subjected to SDS gel electrophoresis and western blotting.
Cell pellets were resuspended and sonicated in lysis buffer (10 mM Tris-HCl, pH 7.5; 150 mM NaCl; 2 mM EDTA; 0.5% Triton X-100; protease inhibitor cocktail) and centrifuged at 15,000 g for 10 min. Five micrograms of anti-Rac1 antibody was added to each sample. After incubation at 4°C for 4 hours, 20 µl of agarose beads conjugated to protein A were added to the supernatant of each sample and incubated with shaking at 4°C for 1 hour. The agarose beads were washed with rinsing buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.05% Triton X-100) six times. Agarose-associated protein complexes were eluted using SDS loading buffer and analyzed by western blots.
IF analyzes.
Cell clusters on polyHEMA-coated plates were processed for immunofluorescence analysis as follows: cells were pelleted by centrifugation at 500 × g for 2 minutes and resuspended in PBS. The clusters were placed on slides, dried briefly and then fixed with 4% paraformaldehyde. Stained samples were imaged using a Spot RT camera attached to a Zeiss upright epifluorescence microscope or a Stanford Photonics XR/Mega-10 ICCD camera attached to a Solamere Technology Group (Salt Lake City, UT) spinning disk confocal system comprised of a Zeiss Axiovert 200M inverted microscope.
Real-time RT-PCR.
Total RNA was extracted from cells using Trizol reagent (Invitrogen). cDNA was synthesized from 0.5 µg of RNA samples using Superscript first strand synthesis kit (Invitrogen). Quantitative real-time PCR analysis was performed with the Lightcycler System using the Lightcycler FastStart DNA Master SYBR Green I kit (Roche) as described previously.Citation11
Abbreviations
| 3D | = | three-dimension(al) |
| co-IP | = | co-immunoprecipitation |
| DG | = | dystroglycan |
| ECM | = | extracellular matrix |
| FAK | = | focal adhesion kinase-1 |
| ILK | = | β1-integrin-linked protein kinase |
| LrECM | = | laminin-rich ECM |
| LN1 | = | laminin-111 |
| MEC | = | mammary epithelial cell |
| polyHEMA | = | poly(2-hydroxyethyl methacrylate) |
| Prl | = | prolactin |
| PrlR | = | prolactin receptor |
| PI3K | = | phosphoinositide 3-kinase |
| STAT5 | = | signal transducers and activators of transcription protein 5 |
Figures and Tables
Figure 1 LrECM induces cell polarization and sustained STAT5 activation. (A) Phase and immunofluorescence image of EpH4 cells on polyHEMA in the presence or absence of LrECM. Immunofluorescence analysis of the basal marker α6-integrin (green), the apical marker ZO-1 (red) and the lateral marker E-cadherin (red) demonstrated that EpH4 cells established apical and basal polarity after LrECM treatment for 24 hours. DAPI nuclear stain is represented as blue. Scale bar: 25 µm. (B) EpH4 cells cultured on polyHEMA were treated with Prl for different time intervals in the presence or absence of 2% LrECM and then analyzed for total and phosphorylated STAT5 levels by western blotting.
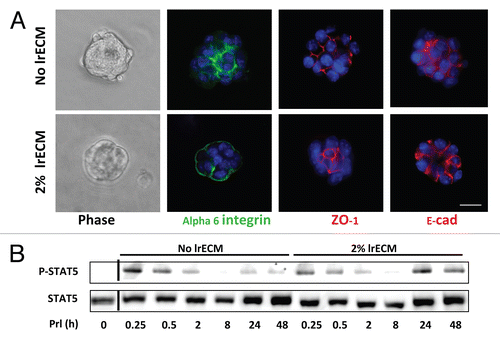
Figure 2 Sustained STAT5 activation is required for induction of the β-casein promoter. (A) Scheme showing the generation of a 16 copy β-casein promoter-CFP construct. (B) EpH4 cells stably transfected with the vector described in (A) were cultured on polyHEMA for 24 hours, and treated for 24 hours with Prl and different ECM molecules as indicated. AG490 was added 8 h after the onset of LrECM and prolactin treatment to specifically block sustained STAT5 activation. Fluorescence microscopy was used to measure the extent of β-casein promoter activation. Cell aggregates expressing the 16 copy-β-casein promoter-CFP construct are white.
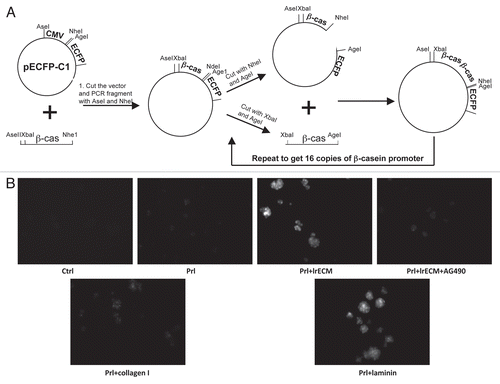
Figure 3 LrECM induces basal localization of PI3K and activation of Rac1. (A) Immunofluorescence analysis of α6-integrin (red) and PI3K (green) in EpH4 cells that were cultured on polyHEMA for 24 hours and then treated with 2% LrECM for another 24 hours. Scale bar: 25 µm. (B) EpH4 cells cultured on polyHEMA were treated with Prl or 2% LrECM plus Prl for 24 hours. After treatment, activated Rac-1 (GTP-Rac1) was isolated using a GST-PBD pull-down assay, and then analyzed by western blotting. The intensity of activated Rac-1 was quantified and normalized to total Rac1 protein levels; *p < 0.05, n = 3. (C) Co-IP analysis of EpH4 cells treated with Prl or 2% LrECM plus Prl showing that LrECM treatment enhances the interaction between STAT5 and Rac1. Protein complexes from EpH4 cell lysates were immunoprecipitated with an anti-Rac1 antibody and the resulting IP was analyzed for STAT5 by western blotting.
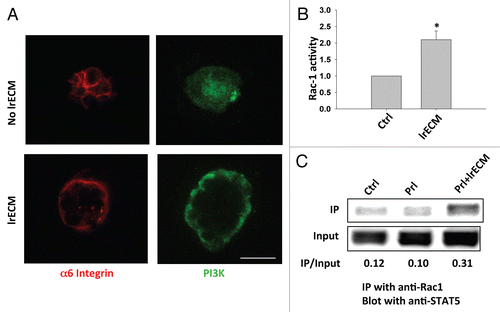
Figure 4 Blocking the PI3K-Rac1 pathway inhibits sustained STAT5 activation and β-casein expression. (A) Western blot analysis showing sustained STAT5 activation in EpH4 cells on polyHEMA that were treated with Prl and 2% lrECM for 8 hours and then LY294002 for an additional 16 hours before harvest. (B and C) Following the treatment as described in (A), GST-PBD pull-down assay was performed to analyze the Rac1 activities (B) and Rac1-STAT5 interaction was detected by co-IP analysis (C). (D) Quantification of β-casein mRNA levels in control and LY294002-treated cells by real-time RT-PCR. β-casein mRNA levels were normalized to 18S levels. (E) Fluoresence microscopy images displaying the extent of β-casein promoter activation in control and LY294002-treated cells. Scale bar: 50 µm.
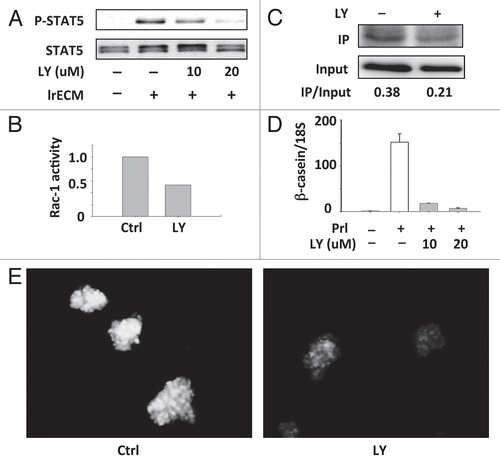
Figure 5 Introducing a constitutively active STAT5 rescues β-casein expression in LY-treated cells. (A) Western blot analysis of STAT5 phosphorylation after LY294002 treatment (10 µM) in STAT5A 1*6-expressing and vector control cells. (B) Real-time RT-PCR measuring β-casein mRNA levels in STAT5A 1*6-expressing and control cells after LY294002 treatment. β-casein mRNA levels were normalized to 18S mRNA levels.
Acknowledgements
We thank Dr. Masahiko Itoh (Dokkyo University Tochigi prefecture, Japan) for providing the ZO-1 antibody. The construct containing STAT5A 1*6 was a kind gift from Dr. Toshio Kitamura (University of Tokyo, Japan). This work was supported by grants from the US Department of Energy, the Office of Biological and Environmental Research (contract no. DE-AC02-05CH1123), a Low Dose Radiation Program and a Distinguished Fellow Award from the Office of Health and Environmental Research Health Effects Division (contract no. 03-76SF00098) to M.J.B., the National Cancer Institute (awards R01CA064786, U54CA126552 and U54CA112970 to M.J.B. and R01CA057621 to M.J.B. and Zena Werb), the US Department of Defense Medical and Materiel Command Innovator Award (contract no.W81XWH0810736 and W81XWH0510338) to M.J.B. and postdoctoral fellowships DAMD17-02-1-0441 to R.X. and W81XWH0410581 to V.A.S. and a post-doctoral fellowship to V.A.S. from the Canadian Institutes of Health Research.
References
- Levy DE, Darnell JE Jr. Stats: Transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002; 3:651 - 662
- Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 2002; 285:1 - 24
- Schmitt-Ney M, Happ B, Ball RK, Groner B. Developmental and environmental regulation of a mammary gland-specific nuclear factor essential for transcription of the gene encoding beta-casein. Proc Natl Acad Sci USA 1992; 89:3130 - 3134
- Watson CJ, Burdon TG. Prolactin signal transduction mechanisms in the mammary gland: the role of the Jak/Stat pathway. Rev Reprod 1996; 1:1 - 5
- Gouilleux F, Wakao H, Mundt M, Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J 1994; 13:4361 - 4369
- Gouilleux F, Pallard C, Dusanter-Fourt I, Wakao H, Haldosen LA, Norstedt G, et al. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. EMBO J 1995; 14:2005 - 2013
- Shillingford JM, Miyoshi K, Robinson GW, Grimm SL, Rosen JM, Neubauer H, et al. Jak2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol Endocrinol 2002; 16:563 - 570
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 1997; 11:167 - 178
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 1997; 11:179 - 186
- Streuli CH, Edwards GM, Delcommenne M, Whitelaw CB, Burdon TG, Schindler C, et al. Stat5 as a target for regulation by extracellular matrix. J Biol Chem 1995; 270:21639 - 21644
- Xu R, Spencer VA, Bissell MJ. Extracellular matrixregulated gene expression requires cooperation of SWI/SNF and transcription factors. J Biol Chem 2007; 282:14992 - 14999
- Xu R, Nelson CM, Muschler JL, Veiseh M, Vonderhaar BK, Bissell MJ. Sustained activation of STAT5 is essential for chromatin remodeling and maintenance of mammary-specific function. J Cell Biol 2009; 184:57 - 66
- Muschler J, Lochter A, Roskelley CD, Yurchenco P, Bissell MJ. Division of labor among the alpha6beta4 integrin, beta1 integrins and an E3 laminin receptor to signal morphogenesis and beta-casein expression in mammary epithelial cells. Mol Biol Cell 1999; 10:2817 - 2828
- Weir ML, Oppizzi ML, Henry MD, Onishi A, Campbell KP, Bissell MJ, et al. Dystroglycan loss disrupts polarity and {beta}-casein induction in mammary epithelial cells by perturbing laminin anchoring. J Cell Sci 2006; 119:4047 - 4058
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol 2005; 171:717 - 728
- Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol 2007; 213:565 - 573
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: Basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol 1991; 115:1383 - 1395
- Liu H, Radisky DC, Wang F, Bissell MJ. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. J Cell Biol 2004; 164:603 - 612
- Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell 1997; 91:949 - 960
- Simon AR, Vikis HG, Stewart S, Fanburg BL, Cochran BH, Guan KL. Regulation of STAT3 by direct binding to the Rac1 GTPase. Science 2000; 290:144 - 147
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression?. J Theor Biol 1982; 99:31 - 68
- Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: Dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev 2009; 28:167 - 176
- Schmidhauser C, Casperson GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ. A novel transcriptional enhancer is involved in the prolactin- and extracellular matrix-dependent regulation of beta-casein gene expression. Mol Biol Cell 1992; 3:699 - 709
- Myers CA, Schmidhauser C, Mellentin-Michelotti J, Fragoso G, Roskelley CD, Casperson G, et al. Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Mol Cell Biol 1998; 18:2184 - 2195
- Rana B, Mischoulon D, Xie Y, Bucher NL, Farmer SR. Cell-extracellular matrix interactions can regulate the switch between growth and differentiation in rat hepatocytes: reciprocal expression of C/EBPalpha and immediate-early growth response transcription factors. Mol Cell Biol 1994; 14:5858 - 5869
- Clayton DF, Harrelson AL, Darnell JE Jr. Dependence of liver-specific transcription on tissue organization. Mol Cell Biol 1985; 5:2623 - 2632
- Lelievre SA, Weaver VM, Nickerson JA, Larabell CA, Bhaumik A, Petersen OW, et al. Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc Natl Acad Sci USA 1998; 95:14711 - 14716
- Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2002; 2:205 - 216
- Kaminker P, Plachot C, Kim SH, Chung P, Crippen D, Petersen OW, et al. Higher-order nuclear organization in growth arrest of human mammary epithelial cells: a novel role for telomere-associated protein TIN2. J Cell Sci 2005; 118:1321 - 1330
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, et al. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol 1995; 129:591 - 603
- Akhtar N, Streuli CH. Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J Cell Biol 2006; 173:781 - 793
- Kawashima T, Bao YC, Nomura Y, Moon Y, Tonozuka Y, Minoshima Y, et al. Rac1 and a GTPase-activating protein, MgcRacGAP, are required for nuclear translocation of STAT transcription factors. J Cell Biol 2006; 175:937 - 946
- Akhtar N, Marlow R, Lambert E, Schatzmann F, Lowe ET, Cheung J, et al. Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation. Development 2009; 136:1019 - 1027
- Nagy T, Wei H, Shen TL, Peng X, Liang CC, Gan B, et al. Mammary epithelial-specific deletion of the focal adhesion kinase gene leads to severe lobulo-alveolar hypoplasia and secretory immaturity of the murine mammary gland. J Biol Chem 2007; 282:31766 - 31776
- Shen TL, Guan JL. Differential regulation of cell migration and cell cycle progression by FAK complexes with Src, PI3K, Grb7 and Grb2 in focal contacts. FEBS Lett 2001; 499:176 - 181
- Qian Y, Zhong X, Flynn DC, Zheng JZ, Qiao M, Wu C, et al. ILK mediates actin filament rearrangements and cell migration and invasion through PI3K/Akt/Rac1 signaling. Oncogene 2005; 24:3154 - 3165
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004; 304:554
- Cotarla I, Ren S, Zhang Y, Gehan E, Singh B, Furth PA. Stat5a is tyrosine phosphorylated and nuclear localized in a high proportion of human breast cancers. Int J Cancer 2004; 108:665 - 671
- Reichmann E, Ball R, Groner B, Friis RR. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J Cell Biol 1989; 108:1127 - 1138
- Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA 1994; 91:12378 - 12382