Abstract
Mechanisms of cAMP/PKA-induced meiotic arrest in oocytes are not completely identified. In cultured, G2/M-arrested PDE3A-/- murine oocytes, elevated PKA activity was associated with inactivation of Cdc2 and Plk1, and inhibition of phosphorylation of histone H3 (S10) and of dephosphorylation of Cdc25B (S323) and Cdc2 (Thr14/Tyr15). In cultured WT oocytes, PKA activity was transiently reduced and then increased to that observed in PDE3A-/- oocytes; Cdc2 and Plk1 were activated, phosphorylation of histone H3 (S10) and dephosphorylation of Cdc25B (S323) and Cdc2 (Thr14/Tyr15) were observed. In WT oocytes, PKAc were rapidly translocated into nucleus, and then to the spindle apparatus, but in PDE3A-/- oocytes, PKAc remained in the cytosol. Plk1 was reactivated by incubation of PDE3A-/- oocytes with PKA inhibitor, Rp-cAMPS. PDE3A was co-localized with Plk1 in WT oocytes, and co-immunoprecipitated with Plk1 in WT ovary and Hela cells. PKAc phosphorylated rPlk1 and Hela cell Plk1 and inhibited Plk1 activity in vitro. Our results suggest that PKA-induced inhibition of Plk1 may be critical in oocyte meiotic arrest and female infertility in PDE3A-/- mice.
Introduction
By catalyzing hydrolysis of cAMP and cGMP, cyclic nucleotide phosphodiesterases (PDEs) are critical regulators of the intracellular concentrations and, consequently, the biological effects of these important second messengers. PDEs belong to a complex and diverse superfamily which include at least 11 structurally related gene families (PDEs1-11).Citation1 The PDE3 gene family is composed of two subfamilies, PDE3A and PDE3B. PDE3 enzymes hydrolyze cAMP and cGMP with high affinity in a mutually competitive manner, are selectively inhibited by several compounds, including milrinone, cilostamide and cilostazol, and are phosphorylated and activated in different types of cells in response to insulin and IGF1, as well as to agents that increase cAMP.Citation2
Although competent to complete meiosis, mammalian oocytes are physiologically arrested in prophase I (prophase of the first meiotic division) until shortly before ovulation. Meiotic arrest and resumption require the integration of signaling pathways and communication networks in oocytes and surrounding ovarian cumulus granulosa cells.Citation3,Citation4 In murine oocytes, cAMP, most likely via protein kinase A-catalyzed (PKA-catalyzed) phosphorylation of downstream effectors,Citation5,Citation6 including Cdc25BCitation7–Citation9 and Wee 1 kinase,Citation10 inhibits activation of maturation-promoting factor (MPF), which consists of Cdc2 in complex with Cyclin B, and thereby inhibits oocyte maturation and maintains meiotic arrest. Elevated intraoocyte cAMP concentrations are most likely maintained by constitutively active oocyte G protein-linked receptors which activate oocyte adenylyl cyclase, and by cGMP-mediated inhibition of oocyte PDE3A which results from diffusion of cGMP into oocytes from surrounding cumulus cells through gap junctions.Citation11,Citation12 PDE3A, relatively highly expressed in mammalian oocytes, is the predominant PDE responsible for hydrolysis of oocyte cAMP.Citation13,Citation14 Current thinking suggests that resumption of meiosis is triggered by LH, which increases cAMP and reduces cGMP in cumulus cells, leading to closure of gap junctions and decrease in oocyte cGMP. This relieves cGMP-induced inhibition of oocyte PDE3A, resulting in PDE3A-induced hydrolysis of oocyte cAMP,Citation14,Citation15 and resumption of meiotic maturation.Citation3,Citation4,Citation11,Citation12 PDE3A-/- female mice are sterile, most likely due to increased oocyte cAMP content, leading to activation of PKA and subsequent PKA-induced meiotic block at the G2/M transition.Citation14
The transition from G2 to meiosis depends on activation of MPF, which is controlled by phosphorylation/dephosphorylation events and changes in subcellular localization. During G2 arrest, phosphorylation of Cdc2 at Thr14 (by Myt1) and Tyr15 (by Wee1 and Myt1) results in inactivation of Cdc2/cyclin B1 (MPF).Citation16–Citation19 Activation of Cdc2 is controlled at several steps, including phosphorylation of threonine 161 by CDK-activating kinase (CAK) complexCitation20,Citation21 and dephosphorylation of Cdc2 Thr14 and Tyr15, catalyzed by the Cdc25 dual-specificity phosphatase family (Cdc25A, B and C).Citation22,Citation23 Cdc25B, critical in Cdc2-dephosphorylation at the G2/M transition, was found to be essential for meiotic resumption in female mice.Citation24 Although not essential for meiotic progression, siRNA knockdown of Cdc25A in mouse oocytes suggested an important role for this phosphatase in meiotic resumption and spindle formation.Citation25 cAMP and PKA inhibitory effects on MPF activation are indirect, mediated by direct phosphorylation/activation of Wee1 kinaseCitation10 and phosphorylation/inhibition of Cdc25B;Citation7–Citation9 these enzymes, in large part, determine the phosphorylation state of the MPF complex. The differential translocalization of Wee1, Myt1 and Cdc25 between nuclear and cytoplasmic compartments is also important for resumption of meiosis in mouse oocytes.Citation26
Polo-like kinases are involved in a broad range of cell cycle regulatory processes during mitosis and meiosis, including spindle organization, activation of MPF, chromosome segregation, M-phase entry, centrosome maturation and M-phase exit.Citation27,Citation28 Polo-like kinase 1 (Plk-1), inactive during G2 arrest, becomes activated at the same time as MPF during resumption of meiosis.Citation29 An upstream regulator of Cdc25B and C, Plk1 can stimulate Cdc25B-induced mitosis in U2Os cells by promoting nuclear translocalization of Cdc25B.Citation30 Plk1 phosphorylates/activates Xenopus and human Cdc25C.Citation31–Citation33 Although Plk1 is required for activation of Cdc25C and MPF and initiation of meiotic resumption in Xenopus oocytes,Citation32 it is not certain if Plk1 acts as a trigger kinase for activation of Cdc25B and MPF in mouse oocytes. Activated Plk1 phosphorylates/inactivates Myt1, maintaining its inhibition during meiotic progression.Citation34–Citation37 Also, Plk1 promotes nuclear translocation of Cdc25C, and via phosphorylation of Cyclin B1, can regulate subcellular localization of the Cdc2/cyclin B1 complex.Citation38,Citation39 Little is known, however, about the upstream regulation of Polo-like kinase.
In this report, we investigated activity and phosphorylation status, subcelluar localization and gene expression of several key cell cycle regulators in WT and PDE3A-/- oocytes. Taken together, our results suggest that, in PDE3A-deficient oocytes, increased cAMP/PKA signaling prevents dephosphorylation/activation of MPF and maintains meiotic arrest, perhaps, at least in part, via PKA- catalyzed phosphorylation/inactivation of Cdc25BCitation7–Citation9 and Plk1. Taken together, our studies in PDE3A-/- mice also support the idea that PDE3A and key PKA substrates such as Cdc25B and Plk1 might provide targets for contraceptive drugs.Citation40
Results
Genotyping.
As seen in , WT, PDE3A-/- and heterozygous genotypes were identified by PCR; ∼213 bp fragment was amplified from WT DNA, and an ∼487 bp fragment, from PDE3A-/- DNA. Both fragments were amplified from heterozygous DNA. western blot results confirmed that PDE3A is absent in PDE3A-/- ovary (). As seen in , cultured PDE3A-/- oocytes were arrested at G2/M phase, even after 16 hours incubation.
PKA activity is increased in PDE3A-/- oocytes.
Meiotic arrest in PDE3A-/- oocytes () is most likely due to increased cAMP/PKA signaling which results from the absence of PDE3A, the predominant cAMP-hydrolyzing PDE in mouse oocytes.Citation13,Citation14 We therefore compared PKA activities in WT and PDE3A-/- oocytes. As shown in , in the presence of excess cAMP (2 µM), total PKA activity is similar in lysates from fresh WT and PDE3A-/- oocytes, suggesting that PKA catalytic subunit content is similar in WT and PDE3A-/- oocytes. Basal PKA activity (which reflects the endogenous cAMP content of the oocyte lysates) is significantly increased in PDE3A-/- oocytes, consistent with our earlier report, which demonstrated that cAMP content was increased in PDE3A-/- oocytes, compared to WT oocytes.Citation14 The time-course of changes in PKA activity in cultured PDE3A-/- oocytes and WT oocytes treated with or without the specific PDE3 inhibitor, cilostamide (10 µM), is shown in . In WT oocytes, PKA activity was reduced for ∼1 h, and then increased over the next several hours. In PDE3A-/- oocytes and WT oocytes exposed to cilostamide, PKA activity was elevated during the 5 h incubation. These results suggested that in WT oocytes, a temporary reduction in PKA activity is critical for G2/M transition, and that reactivated PKA did not restrict the cell cycle once meiosis progression had traversed G2/M. In PDE3A-/- oocytes and WT oocytes exposed to cilostamide, prophase arrest was maintained, most likely due to increased cAMP and increased PKA activity. This data also was consistent with our previous study which demonstrated that incubation of PDE3A-/- ooyctes with PKA inhibitors for 1 h was sufficient to release PDE3A-/- oocytes from meiotic blockade and trigger germinal vesicle breakdown (GVBD).Citation14
Confocal microscopic localization of PKA catalytic subunits (PKAc) in oocytes.
Meiotic resumption in denuded WT oocytes was associated with development of a well-defined spindle apparatus with condensed and aligned chromosomes typical of metaphase. In contrast, PDE3A-/- oocytes did not resume meiosis in vitro and remained blocked at GV stage even after incubation for 18–24 h.Citation14 As shown in , in freshly isolated WT oocytes, PKAc were detected in cytoplasm, and some PKAc colocalized with pericentrin. During GVBD, within ∼1 h, PKAc entered the nucleus and became localized at the spindle apparatus. In PDE3A-/- oocytes, since GVBD does not occur, PKAc remained in cytoplasm and apparently colocalized with pericentrin, even after incubation for 4 h.
Histone H3 is not phosphorylated at Ser10 in PDE3A-/- oocytes.
It has been suggested that phosphorylation of histone H3 at serine 10 is critical for initiation of chromosome DNA condensation during mitosis and meiosis.Citation41–Citation43 Salvador and his colleagues found that follicle-stimulating hormone stimulated protein kinase A-mediated histone H3 phosphorylation at serine 10 in ovarian granulosa cells.Citation44 As shown in (H3 part), histone H3 became markedly condensed (within 4 h) as meiosis proceeded in cultured WT oocytes; chromosome condensation did not occur in PDE3A-/- oocytes. Histone H3 phosphorylation (Ser10) was minimal in freshly isolated oocytes, but, within 4 h, histone H3 phosphorylation (Ser10) (and chromosomal condensation) were markedly increased in cultured WT, but not PDE3A-/- oocytes or WT oocytes treated with the PDE3 inhibitor, cilostamide. These results were recapitulated in western blots. As shown in , phosphorylation of histone H3 at serine 10 was detected in lysates of cultured (4 h) WT oocytes, but not PDE3A-/-, oocytes, perhaps because PKAc did not translocate to the spindle apparatus in PDE3A-/- oocytes.
Inactivation of Cdc2 in PDE3A-/- oocytes.
Activation of MPF (Cdc2/cyclin B1) is the key molecular event in initiation of meiosis.Citation5,Citation6,Citation29 As seen in , Cdc2 activity markedly increased during spontaneous maturation of cultured WT oocytes (), but was not activated in cultured WT oocytes exposed to cilostamide or PDE3A-/- oocytes, which did not undergo GVBD (). Activation of Cdc2 is dependent on dephosphorylation at Thr14 and Tyr15, and as shown in , western Blot analysis indicated that Cdc2 was dephosphorylated at Thr14/Tyr15 to a much greater extent in WT ooyctes than in PDE3A-/- oocytes. Complete activation of MPF also requires CAK-induced phosphorylation of Cdc2 at Thr161, and Cyclin B1 synthesis. As seen in , Cdc2 was phosphorylated at Thr161 both in WT and PDE3A-/- oocytes, suggesting the CAK activity was not altered in PDE3A-/- oocytes.
Subcellular localization of Cdc2/Cyclin B1 in PDE3A-/- oocytes.
Subcellular trafficking plays an important role in MPF-induced resumption of meiosis in oocytes.Citation45 As seen in , in WT oocytes, Cdc2 was widely distributed throughout the cytoplasm and nucleus; during GVBD it localized to the spindle apparatus. Some phospho(Thr14/Tyr15)-Cdc2 [p-Cdc2(T14/Y15)] seems to be localized at centrosomes (microtubule organizing centers (MTOCs) in murine oocytes) in fresh WT oocytes, but the signal was markedly reduced at 4 h (at the spindle poles) and very weak after 18 h incubation. In cultured PDE3A-/- oocytes, p-Cdc2(T14/Y15) remained in the nucleus and at MTOCs.
Taken together, results in are consistent with the recognized association of Cdc2 with centrosomes, which are thought to be a platform for dephosphorylation of Cdc2 and activation of MPF,Citation56,Citation71–Citation73 as well as with the spindle apparatus.Citation45,Citation74,Citation75 Cdc2 phosphorylates the tumor suppressor WARTS, a protein which associates with mitotic spindles and spindle poles, and regulates cell cycle progression via its interaction with zyxin, a regulator of actin filament assembly.Citation76 Cdc2 is also an important regulator of the spindle checkpoint by phosphorylation of inhibitors (Emi2, BubR1 and Cdc20) of the anaphase promoting complex (APC); inactivation of Cdc2 regulates, in part, dephosphorylation of these proteins, accumulation/activation of the APC, and the triggering of anaphase.Citation77–Citation79
In vertebrate cells, the nuclear entry of Cdc2/Cyclin B1 during prophase is thought to be essential for the induction and coordination of M-phase events. Phosphorylation of Cyclin B1 is central to its nuclear translocation.Citation39,Citation46 As seen in , in both freshly isolated WT and PDE3A-/- oocytes, immunofluorescent Cyclin B1 was detected primarily in the cytoplasm, with some Cyclin B1 detected at MTOCs. Within 1 h, Cyclin B1 transferred to nucleus in cultured WT oocytes, but not PDE3A-/-, oocytes. Within 4 h, WT oocytes underwent GVBD, immunofluorescent Cyclin B1 was detected at the spindle apparatus and centrosomes, but in cultured PDE3A-/- oocytes, the distribution of Cyclin B1 immunofluorescence changed very little during incubation (). Expression of immunoreactive Cyclin B1 seemed to transiently increase and then decrease in PDE3A-/- oocytes ().
A critical step in activation of MPF is accumulation of sufficient Cyclin B to form Cyclin B/Cdc2 complexes. In mouse oocytes the concentration of Cyclin B exceeds that of Cdc2, and it is possible that inactive Cyclin B/Cdc2 complexes are preassembled in arrested oocytes prior to activation of MPF and reentry into the meiotic cell cycle.Citation6,Citation34 Taken together, our results suggested that dysregulation of dephosphorylation of MPF (), and not reduced expression of Cyclin B (), was primarily responsible for deficient activation of MPF and meiotic arrest in PDE3A-/- oocytes. The transient increase in Cyclin B in PDE3A-/- oocytes may be a compensatory response to inactive MPF, although there have been reports of irradiation- and herpesvirus 68 (MHV68)-induced G2/M arrest being associated with increased Cyclin B levels and inactive Cdc2.Citation80–Citation82
Subcellular localization of Cdc25B protein phosphatase in PDE3A-/- oocytes.
In mammalian cells, Cdc25B-induced dephosphorylation/activation of Cdc2/Cyclin B1 complexes is necessary for entry into meiosis.Citation24 In cultured rat oocytes, Cdc25B, whose translation is regulated by polyadenylation of its mRNA, exhibits periodic accumulation and reduction during reinitiation of meiosis, with a marked, transient, reduction at metaphase I.Citation47 Gabrielli and co-workers found that endogenous Cdc25B was nuclear in early G2 phase but translocated to the cytoplasm just before centrosomal nucleation in prophase.Citation48 Consistent with those reports, as seen in , in cultured WT oocytes, Cdc25B was translocated from nucleus to cytoplasm within 1 h, and its immunofluorescent signal was markedly reduced within 4 h. In cultured PDE3A-/- oocytes, however Cdc25B was readily detected in nucleus, even after 4 h, perhaps due to alterations in mRNA polyadenylation or translation of Cdc25B.Citation47 Murine Cdc25B Ser-321 (homologous to human Ser-323, and when phosphorylated, is recognized by anti-phospho-Ser-323) can be phosphorylated by PKACitation8,Citation9 and is a primary 14-3-3 binding site, which influences activity and cellular localization of Cdc25B.Citation23 As seen in , after incubation for 1 h, immunoreactive phospho-Cdc25B (S321) was readily detected in cultured PDE3A-/- oocytes, but not WT oocytes, consistent with increased PKA-induced phosphorylation in PDE3A-/- oocytes. These results suggest that dysregulation of Cdc25B localization and activation may be important in inhibition of MPF activation and maintenance of meiotic arrest in PDE3A-/- oocytes.
Wee1 and checkpoint kinase activities were similar in WT and PDE3A-/- oocytes.
Wee1 phosphorylates Cdc2 at Tyr15 and inhibits MPF activity.Citation6,Citation10 As seen in , in cultured PDE3A-/- oocytes or WT oocytes treated without or with cilostamide, Wee1 activity in oocyte lysates decreased during 5 h incubation, suggesting that changes in Wee1 may not be critical in meiotic prophase arrest of PDE3A-/- oocytes.
Cell cycle checkpoints are the signal transduction pathways that couple the detection of DNA damage to the proteins that control cell cycle transitions. In addition, Cdc25B and Cdc25C are negatively regulated by phosphorylations induced by Check point kinases, Chk1 and Chk2.Citation23,Citation49 As seen in , Check point kinase activities were similar in cultured WT and PDE3A-/- oocytes, suggested that changes in check point kinase were not responsible for meiotic prophase arrest of PDE3A-/- oocytes.
Polo-like kinase gene expression is altered in PDE3A-/- oocytes.
Of eight cell cycle-related genes (>1.5-fold change) amplified in each of two sequential rounds of amplification, and identified by Affymetrix microarray analysis, five genes were related to polo like kinase (). For example, compared with WT oocytes, expression of mRNA of polo-like kinase 2 increased ∼3.8 fold in PDE3A-/- oocytes; Polo-like kinase 1(Plk1), which plays key role in G2/M transition, increased ∼1.6-fold; Cell division cycle 5-like (Cdc5l), which is similar in structure and function to polo-like kinase, increased ∼3.1-fold. Expression of mRNA of Cep164, a novel centriole appendage protein and a key player in G2/M checkpoint and nuclear divisions,Citation50 was increased ∼7.6-fold in PDE3A-/- oocytes; Pard3, which is thought to be a regulator of polo-like kinase and Cdc25,Citation51 was increased ∼2.4.fold in PDE3A-/- oocytes. Expression of other cell cycle regulatory genes which were critical for G2/M phase transition, such as Cdc2, Cyclin B1, Cdc25, Check point kinase, p53, Wee1 and Myt1, was not altered in PDE3A-/- oocytes.
To verify the microarray data, protein expression was assessed in western blots. As seen in , consistent with gene chip data, immunoreactive Plk1 and Cdc5l, as well as p-CREB(Ser133), were increased in PDE3A-/- oocytes compared to WT oocytes. On the other hand, expression of immunoreactive p53, Cdc25B, Cdc25C, Cdc2, Cyclin B1, Wee1, Chk1 and Chk2 were similar in WT and PDE3A-/- oocytes. These data suggest that polo-like kinase related gene expression was increased in PDE3A-/- oocytes.
PKA-induced inactivation of Plk1 in PDE3A-/- oocytes and of recombinant Plk1 (rPlk1) in vitro.
Plk1 is an important regulator of activation of Cdc25, MPF and meiotic progression in Xenopus oocytes.Citation32 As seen in , during spontaneous maturation of cultured WT oocytes, Plk1 activityCitation52 rapidly increased within the same time frame as Cdc2 (cf ) and was maximal within ∼1 h incubation, but in arrested PDE3A-/- oocytes, Plk1 activity did not increase, even though immunoreactive Plk1 was somewhat increased as compared to WT oocytes (cf ). As seen in , in WT oocytes, activation of Plk1 could be inhibited by PDE3 inhibitor cilostamide in WT oocytes; in PDE3A-/- oocytes, Plk1 could be reactivated by treatment with the PKA inhibitor. These results suggest that activation of Plk1 is regulated by cAMP/PKA signaling; in PDE3A-/- oocytes, Plk1 activity is inhibited, perhaps due to the increase in oocyte cAMP and consequent activation of PKA.
As seen in , PKA, in a concentration-dependent manner, phosphorylates rPlk1 in vitro, and inhibits its activity, suggesting that Plk1 could be a substrate of PKA in vivo. Although it is not certain that PKA directly phosphorylates Plk1 in vivo, as seen in , co-immunoprecipitation experiments demonstrated that both PDE3A and Plk1 were present in immunocomplexes from mouse ovary or Hela cell lysates, after pull-downs with either anti-PDE3A or anti-Plk1 antibodies. PKA also phosphorylates endogenous Plk1 immunoprecipitated from Hela cells and inhibits its activity (). In Hela cell lysates, Plk1 co-immunoprecipitated with PKAc, Cdc25C, PDE3A and PP2A, suggesting that Plk1 is a component of macromolecular complexes involved in cAMP/PKA signaling pathways in Hela cells (and, by implication, murine oocytes).
Localization of PDE3A and Plk1 in oocytes.
As seen in , in freshly isolated WT oocytes, Plk1 was detected primarily in the cytoplasm and at MTOCs. During spontaneous maturation of cultured WT oocytes, Plk1 entered into the nucleus (within 1 h).
Within 4 h, WT oocytes demonstrated GVBD, and Plk1 was detected at the spindle apparatus and MTOCs. PDE3A partially co-localized with Plk1 at the spindle apparatus in WT oocytes. In PDE3A-/- oocytes, no PDE3A signal was detected, and Plk1 was exclusively located in the cytoplasm, not in the nucleus, even after 4 h incubation.
Discussion
In murine oocytes, cAMP, most likely via PKA-catalyzed phosphorylation of downstream targets and effectors, prevents activation of MPF, and thereby inhibits oocyte maturation and maintains physiologic meiotic arrest.Citation5,Citation6,Citation9,Citation14,Citation29 The transition from G2 arrest to meiosis requires a transient reduction in cAMP (due, in part, to hydrolysis by PDE3A15) and in cAMP/PKA signaling.Citation3–Citation6,Citation29 PDE3A-/- female mice are sterile, most likely due to increased oocyte cAMP content, which leads to PKA-induced meiotic blockade.Citation14 These mice provide a model system to investigate cAMP/PKA regulation of the G2/M transition.
Activity of MPF (Cdc2/Cyclin B1), the key regulator of G2/M transition and resumption of meiosis in oocytes, is tighly regulated by phosphorylation and dephosphorylation events and changes in subcellular localization.Citation5–Citation10,Citation26,Citation53,Citation54 In cultured WT oocytes, activation of Cdc2 was initiated within 1 h (), at a time when PKA activity was transiently reduced (). Once Cdc2 activation was initiated, reactivation of PKA did not block activation of Cdc2, suggesting, as in Xenopus oocytes,Citation55 a critical temporal window for inhibition of Cdc2 by PKA in WT oocytes. We previously reported that brief exposure (∼1 h) of PDE3A-/- oocytes to a PKA inhibitor was sufficient to trigger resumption of meiosis and activation of Cdc2/CyclinB and MAPK.Citation14 In WT oocytes, activation of Cdc2/Cyclin B1 and GVBD was associated with dephosphorylation of Cdc2 at Thr14/Tyr15, presumably catalyzed by Cdc25B ().Citation24 In contrast to WT oocytes, in cultured PDE3A-/- oocytes, Cdc2 was not dephosphorylated or activated ( and B), suggesting that PKA-mediated inhibition of Cdc25B plays an important role in meiotic arrest in PDE3A-/- oocytes. Microinjection of protein kinase inhibitor peptide (PKI) or mRNA encoding Cdc25 into PDE3A-/- oocytes resulted in resumption of meiosis.Citation14 Full activation of Cdc2 also requires Thr161 phosphorylation by Cdk-activating kinases (CAK).Citation20,Citation21 Our results indicated that Cdc2 was phosphorylated at Thr161 in WT and PDE3A-/- oocytes, suggesting that CAK dysregulation was not critical in inhibition of Cdc2 in PDE3A-/- oocytes.
Many proteins or protein complexes that are involved in regulation of cell cycle progression, i.e., Cdc2/Cyclin B1, Cdc25B, Chk1, Chk2, Plk1, PKARII, PKAc, A-Kinase Anchoring Proteins (AKAPs), etc., are apparently associated with MTOCs, which may function as a platform for activation of MPF.Citation56–Citation59 During mitosis, it has been suggestedCitation49 that centrosome-associated Chk1 (or perhaps PKACitation8,Citation9), via phosphorylation/inhibition of Cdc25B, prevents premature activation of Cdc2. Our results () suggest that, in WT oocytes, resumption of meiosis, i.e., activation of Cdc2, is correlated with apparent dissociation of PKAc from pericentrin and its translocation into the nucleus, where it may phosphorylate histone H3(Ser 10), which is thought to play an important role in initiation of mammalian chromosomal DNA condensation.Citation41–Citation44,Citation59 During mitosis, activated Cdc2 has been reported to phosphorylate PKARII, causing its dissociation from centrosomes, perhaps because of its decreased affinity for AKAP450.Citation60 In prophase-arrested PDE3A-/- oocytes, however, PKAc remains associated with pericentrin, and does not enter the nucleus, and H3 phosphorylation and DNA condensation were not observed in cultured PDE3A-/- oocytes. It is not known how, or if, translocation of PKAc is involved in the transient activation of PDE3A, reduction in cAMP and PKA activity, and activation of MPF that characterizes resumption of meiosis. Taken together, these findings suggest that PKA most likely regulates meiosis through specific and highly regulated temporal and spatial interactions with other signaling pathways and modules. PKAs are most likely targeted to specific oocyte subcellular compartments/microdomains by specific AKAPs or AKAP-like proteins which function as molecular scaffolds, placing PKA in close proximity to both physiological substrates as well as upstream regulators and downstream effectors and targets.Citation9,Citation61,Citation62
Although PKA does not directly phosphorylate Cdc2, Wee1 kinase and Cdc25B are two important PKA targets that directly regulate activation of Cdc2Citation6,Citation9,Citation10,Citation17,Citation26 (). PKA-induced activation of Wee1 kinase results in phosphorylation of Cdc2 Tyr15, and prevents dephosphorylation/activation of Cdc2 by Cdc25B.Citation6,Citation10,Citation16–Citation19 On the other hand, Cdc25B is inactivated by PKA-induced phosphorylation.Citation7–Citation9 Thus, in WT oocytes, transient activation of PDE3A is thought to reduce cAMP/PKA-signaling, resulting in reduced inhibitory phosphorylation of Cdc2 by Wee1 kinase and enhanced dephosphorylation/activation of Cdc2 by activated Cdc25B. Our results indicated that in cultured WT and PDE3A-/- oocytes, Wee1 activity rapidly decreased, suggesting that dysregulation of Wee1 may not be involved in meiotic arrest of PDE3A-/- oocytes. Although we have not been able to develop a reproducible and reliable assay for Cdc25B in murine oocytes, our results do suggest that inhibition of dephosphorylation of Cdc2 () in PDE3A-/- oocytes correlates with inhibition of dephosphorylation of Cdc25B (), perhaps related to activated PKA ( and B).
Our results also suggest that, in oocytes, another important PKA target may be Plk1 (). In PDE3A-/- oocytes, expression of polo-like kinase 1 mRNA () and protein (immunoreactivity) (), as well as of other genes such as Polo-like kinase 2 and Cdc5l, is increased, suggesting that polo-like kinase family might be involved in regulation of oocyte maturation. Although Plk1 () and Cdc2 () activities increased rapidly (1 h) in WT oocytes, Plk1 activity was lower in PDE3A-/- oocytes than in WT oocytes. The possibility that Plk1 was inhibited by cAMP/PKA signaling was supported by the observations that Plk1 was activated by incubation of PDE3A-/- oocytes with a PKA inhibitor, and inhibited by incubation of WT oocytes with cilostamide, a specific PDE3 inhibitor (). In PDE3A-/- oocytes, Cyclin B1, a Plk1 substrate which translocates to the nucleus after phosphorylation by Plk1,Citation39 was mostly localized in cytoplasm, even after 4 h (), consistent with reduced Plk1 activity in PDE3A-/- oocytes. PDE3A was co-immunoprecipitated with Plk1 in mouse ovary and Hela cell lysates ( and D), and also co-localized with Plk1 in WT oocytes (). In vitro experiments demonstrated that recombinant Plk1 () and Plk1 immunoprecipitated from Hela cells () were phosphorylated and inhibited by PKA, consistent with activation of Plk1 during incubation of PDE3A-/- oocytes with PKA inhibitor (). Previous workers have also reported that the heat stable inhibitor of PKA, PKI, caused activation of Plx1 in oocyte extracts.Citation32 On the other hand, Kelm and associates reported that PKA can phosphorylate and activate Plk1.Citation63 At this point, we cannot explain why these results differ from our findings and those of others.Citation32
We suggest that PKA may also block activation of Cdc2/Cyclin B1 via another downstream target, i.e., Plk1 (). Plk1 has been reported to phosphorylate/inhibit Myt1 kinase,Citation36,Citation37,Citation64,Citation65 which catalyzes inhibitory phosphorylations of Cdc2Thr14/Tyr15.Citation10,Citation16–Citation19 Plk1 also promotes nuclear entry of Cdc25B (perhaps via phosphorylation of an N-terminal domain)Citation30 and Cdc25C,Citation38 and also phosphorylates/activates Cdc25C.Citation31–Citation33 Although Plk1 may activate Cdc25C and trigger resumption of meiosis in Xenopus oocytes,Citation32 it is not certain that Plk1 initiates activation of Cdc25 in murine oocytes. Plk1 may activate Cdc25 as part of a “feed-forward” amplification loop catalyzed by activated MPF, i.e., activated Cdc2 inhibits Wee1 and Myt1 and activates Cdc25B and Plk1 (which further activates Cdc25 and inhibits Myt1).Citation27,Citation34–Citation37,Citation64 Thus, in PDE3A-/- oocytes, PKA-induced phosphorylation/inactivation of Plk1 may result in activation of Myt1 kinase and consequent inhibition of Cdc2. Activation of Plk1 (via inhibition of cAMP/PKA signaling and/or activated Cdc2) in turn activates Cdc25 and inhibits myt1,Citation34,Citation37 resulting in or maintaining activation of Cdc2.
Plk1 is a pleiotropic regulator of cell cycle progression; its C-terminal polo box domain (PBD) is a phosphopeptide binding motif, which allows Plk1 to dock with specific phosphorylated protein targets at different subcellular locations.Citation27,Citation64 Plk1 is thought to be associated with centrosomes and involved in centrosomal function.Citation27,Citation28 The mammalian transcription factor Foxhead Box M1 (FoxM1) regulates expression of a number of genes important for G2/M transition and cell cycle progression; Plk1 modulates this transcriptional network via phosphorylation/activation of FoxM1.Citation65 Thus, dysregulation of Plk1 could affect resumption of meiosis at many levels, including effects on formation of the spindle apparatus,Citation66,Citation67 microtubule assembly,Citation68 MTOC formation and function,Citation69 and expression of critical cell cycle regulatory genes.Citation65
Taken together, our data suggest that Plk1 is a potential cAMP/PKA substrate/target and a component of a macromolecular complex that contains PDE3A and regulates cAMP/PKA-signaling pathways important in progression of meiosis and female infertility. PDE3A-/- mice may provide a model system to help dissect these cAMP/PKA signal transduction networks, with the possibility of identifying rational and effective contraceptive targets.
Materials and Methods
Genotyping.
WT and PDE3A-/- C57BL/6J mice (8 generations back cross) used in this study were maintained in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. DNA was extracted from tails of WT and PDE3A-/- female mice (3∼4 weeks old), as previously described in reference 14; their genotypes were identified by PCR with the following primers: m13-forward: 5′-GCG GTG CTA TAC AAC GAC CGT TCT GTT CTG GAG AAC CAT-3′; m13-Reverse: 5′-CTT GGC GTT AAA TTT GGC TAC AAA GTC AAA GTG TTT CTT C-3′. NEO-forward: 5′-GAT GGC TGG CAA CTA GAA GG-3′; NEO-Reverse: 5′-CAT ACG CTT GAT CCG GCT AC-3′. PCR was conducted using an Advantage®-GC cDNA PCR kit according to the manufacturer's instructions: samples were denatured (95°C, 2 minutes), subsequently cycled at 95°C, 30 seconds; 52°C, 30 seconds; 68°C, 90 seconds for 40 cycles, with final extension for 7 minutes. PCR products were analyzed by electrophoresis in 1.5% agarose gels.
Oocyte collection and culture, synchronization of cultured hela cells, and preparation of mouse ovary lysates.
WT and PDE3A-/- female mice (3–4 weeks old) were sacrificed by asphyxiation (CO2). Ovaries were placed in maturation medium containing DMEM (Invitrogen Crop., Cat#11995-065), 25 mM Hepes (Cellgro, Cat#25-060-CI), 3 mg/ml BSA (Sigma-Aldrich, Cat#A9418), 100 units/ml penicillin (Invitrogen Crop., Cat#15140), 100 µg/ml streptomycin (Invitrogen Crop., Cat#15140), and 10 µM cilostamide (a specific PDE3 inhibitor which blocks spontaneous maturation of cultured WT oocytes). Ovaries were punctured with a fine needle to release oocytes from the antral follicles, and oocytes were quickly harvested with a pulled Pasteur pipette, and then washed 5 × 5 minutes in maturation medium without cilostamide. The meiotic stages of cultured oocytes were followed using a dissecting stereomicroscope (model Stemi SV6; Carl Zeiss Inc., Thornwood, New York, USA). Oocytes showing clear nuclear membranes (germinal vesicles) and nucleoli were classified as GV stage; those without visible nuclear structure and exhibiting germinal vesicle dissolution or breakdown were classified as GVBD stage; and metaphase II-arrested oocytes, with polar bodies, were classified as PB stage.Citation14,Citation40 For experiments, cultured oocytes were incubated for indicated times in maturation medium in the presence or absence of cilostamide (10 µM) at 37°C under 95% O2/5% CO2. At indicated times, cultured oocytes from WT and PDE3A-/- mice were collected and lysed in buffer containing 1% NP-40, 50 mM Tris-HCl pH7.4, 150 mM NaCl, 5 mM EDTA, 1x Protease Inhibitor Cocktail (Pierce, Cat#78410), 2 µM Okadaic acid (Calbiochem, Cat#459620) and 2 µM Calyculin A (Calbiochem, Cat#208851).
Hela cells were cultured in DMEM (Invitrogen Crop., Cat#11995-065) supplemented with 10% FBS and 1% penicillin/streptomycin. For synchronization, Hela cells were arrested at G2/M transition phase by incubation with Nocodazole (400 ng/ml) for 16 hours at 37°C under 95% O2/5% CO2. Mitotic Hela cells were collected by shaking the plate. Cells were homogenized in ice-cold lysis buffer containing 1% NP-40, 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM EDTA, 1x Protease Inhibitor Cocktail (Pierce, Cat#78410), 2 µM Okadaic acid (Calbiochem, Cat#459620) and 2 µM Calyculin A (Calbiochem, Cat#208851).
Mouse ovaries were collected and quickly washed in ice-cold PBS (3 times), then homogenized by OMNI TH Polytron homogenizer (OMNI International, 3 × 5 s, 5,000 rpm) in lysis buffer containing 1% NP-40, 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM EDTA, 1x Protease Inhibitor Cocktail (Pierce, Cat#78410), 2 µM Okadaic acid (Calbiochem, Cat#459620) and 2 µM Calyculin A (Calbiochem, Cat#208851). Lysates were centrifugated (26,000 g, 15 min) at 4°C, supernatant fractions were collected and quickly frozen in −80°C.
PKA, Cdc2, Wee1 and checkpoint kinase activity assays.
PKA activity in oocyte lysates was quantified with a PKA Assay Kit (Upstate, Cat#17-134), using [γ-32P]-ATP (10 µCi/assay) and 0.08 mM of Kemptide as a substrate. Labeled phosphorylated substrate was captured by P81 membranes and radioactivity was quantified by liquid scintillation counting. Cdc2 activity was measured using SignalTECT® Cdc2 Protein Kinase Assay System (Promega, Cat#V6430), using [γ-32P]-ATP (1 µCi/assay) and a biotinylated-peptide derived from histone H1 to serve as substrate for Cdc2 kinase in cell lysates. This peptide (PKT PKK AKK L) (25 µM/assay) is a highly selective substrate for Cdc2 kinase. The phosphorylated substrate and then was recovered from the reaction mix with the SAM2® Biotin Capture Membrane, and radioactivity quantified by liquid scintillation counting. Wee1 kinase activity was measured with CycLex® Wee1 kinase Assay/Inhibitor Screening Kit (MBL, Cat#CY-1172), using wells pre-coated with a substrate corresponding to recombinant Cdc2 which contains a tyrosine residues that can be specifically phosphorylated by Wee1. The amount of phosphorylated substrate is measured by incubating the wells with an anti-Phospho-Tyrosine primary monoclonal antibody, and then with HRP (horseradish peroxidase)-conjugated anti-IgG secondary antibody, which then catalyzes the color conversion. Checkpoint kinase activity was measured with CycLex® Checkpoint kinase Assay/Inhibitor Screening Kit-1 (MBL, Cat#CY-1162), which utilizes a phospho-specific primary monoclonal antibody to recognize phospho-serine 216 residue in Cdc25C, which is phosphorylated by checkpoint kinases. The amount of phosphorylated substrate is quantified by incubation with an HRP-conjugate of an anti-phospho-Cdc25C serine 216 specific antibody, which then catalyzes the color conversion. For the last two assays, color is quantitated by spectrophotometry at 450 nm, and reflects the relative amount of Wee1 and checkpoint kinase activities in the samples, respectively.
Briefly, at indicated times, cultured oocytes from WT and PDE3A-/- mice were collected and lysates prepared as described above. The different assays were carried out according to the manufacturers' instructions. Experiments were repeated three times with different batches of oocytes.
Immunofluorescence.
Immunofluorescence was performed as previously described in reference Citation14. Briefly, groups of 30 oocytes were fixed in PBS with 2% paraformaldehyde (1 h, room temperature (RT)), permeabilized with 0.1% Triton X-100 (10 min, RT), and blocked (overnight, 4°C) in PBS containing 10% goat serum and 100 mM glycine. Fixed oocytes were incubated with primary antibody (overnight, 4°C), washed 5 × 5 minutes in blocking solution at RT, then incubated (1 h, RT) with second antibody, Alexa Fluor 488 or 594-conjugated goat anti-mouse or rabbit antibody (Molecular Probes), diluted 1:400 in blocking solution. After washing 5 × 5 minutes at RT, ooyctes were equilibrated with PBS, pH 8.1/glycerol (1:1) for 10 minutes, and mounted in ProLong Gold antifade reagent with DAPI (Molecular Proble, Cat#P36931). The samples were covered with a cover slide and sealed with nail polish. Images were recorded using a Leica TCS Confocal Microscope (40x, 63x). Unless otherwise indicated, all images within a single figure were taken using identical excitation and detection voltages.
Antibodies.
mouse anti-PKA catalytic subunit (PKAc) (BD, Cat#610981, 1:1,000), rabbit anti-Pericentrin (Abcam, Cat#ab4448, 1:2,000), goat anti-histone H3 (Santa Cruz, Cat#sc-8653, 1:100), rabbit anti-phospho-Histone H3 (Ser10) (Santa Cruz Cat#sc-8656-R, 1:100), rabbit anti-phospho-Cdc2 (Thr14/Tyr15) (Santa Cruz, Cat# sc-12340-R, 1:100), mouse anti-Cdc2 (Santa Cruz Cat#sc-8395, 1:100), mouse anticyclin B1(Abcam, Cat#ab72, 1:1,000). Rabbit anti-Cdc25B (Santa Cruz, Cat# sc-326, 1:100), Mouse anti-Plk1 (Millipore, Cat#05-844, 1:500), Rabbit anti-PDE3A antibody [CT (1098TGENQSLDQVPLQHPSEQ1115)] was generated by our lab.
Western blots.
Samples in NuPAGE® LDS Sample Buffer (Invitrogen, Cat#NP0007) were heated (100°C, 5 min), subjected to SDS-PAGE, transfered to PVDF or nitrocellulose membranes, and blocked (4°C, overnight) in PBST (PBS with 0.05% Tween 20) containing 5% non-fat dry milk or 5% BSA. Blots were incubated with primary antibody in blocking buffer (overnight, 4°C), and then with second antibody (1:1,000∼2,000 dilution, 1 h, RT). Signals were detected using SuperSignal® West Femto Maximum Sensitivity Substrate. Immunodetection of endogenous β-actin was utilized to indicate that equivalent amounts of oocyte protein were present in samples added to the SDS PAGE wells/lanes.
Antibodies.
mouse anti-Cdc2 (Abcam, Cat#ab18, 1:1,000), rabbit anti-Cdc2 (Tyr15) (Cell Signaling, Cat#9112, 1:1,000), rabbit anti-Cdc2 (Thr161) (Cell Signaling, Cat#9114, 1:1,000), mouse anti-Cyclin B1 (Abcam, Cat#ab18219, 1:500), rabbit anti-phospho-histone H3 (Ser10) (Santa Cruz, Cat#sc-8656-R, 1:1,000), rabbit anti-histone H3 (Santa Cruz, Cat#sc-10809, 1:1,000), rabbit anti-Cdc25B (Cell signaling, Cat#9525, 1:500), rabbit anti-phosphor-Cdc25B (Ser323) (Abcam, Cat#53103, 1:1,000), mouse anti-Cdc25C (Santa Cruz, Cat#sc-55513, 1:1,000), mouse anti-β-actin (Sigma, Cat#A5441, 1:2,000), mouse anti-p53 (Abcam, Cat#ab26, 1:500), rabbit anti-Chk1 (Cell signaling, Cat#2345, 1:500), rabbit anti-Chk2 (Abcam, Cat#ab8108, 1:500), rabbit anti-Wee1 (Santa Cruz, Cat#sc-325), mouse anti-Cdc5l (Santa Cruz, Cat#sc-81220, 1:1,000), mouse anti-Plk1 (Millipore, Cat#05-844, 1:1,000), Rabbit anti-PDE3A antibody [CT (1098TGENQSLDQVPLQHPSEQ1115)] was generated by our lab.
Immunoprecipitation (IP).
Immunoprecipitations were performed with IP kits (Sigma, Cat#IP50). Briefly, lysate samples (500∼2,000 µg protein), prepared as described above, were incubated with indicated antibodies (overnight, 4°C), and with equal amounts of non-immune mouse or rabbit IgG as controls, with mixing on a rotator platform. Protein G beads (30 µl) were added and incubated (1∼2 h, 4°C). Beads were washed 3∼4 times with IP buffer (containing 1x protease inhibitors and 1x phosphatase inhibitors) and centrifuged. Beads were boiled for 5 min with sample buffer for SDS-PAGE, and eluates subjected to SDS PAGE/western blots.
RNA isolation and amplification for gene expression profiling.
Groups of 250 oocytes were collected and quickly washed (3 times) in PBS, and then placed in RNA Extraction buffer (Arcturus, Cat#KIT0204) for isolation of total RNA, according to the manufacturer's protocol. T7-based RNA amplification was performed on 5 ng of the isolated total RNA using the Riboamp OA 2-round amplification kit in accord with the manufacturer's instructions (Arcturus, Mountain View, California). Briefly, total RNA was incubated with oligo dT/T7 primers and reverse-transcribed into double-stranded cDNA. In vitro transcription of purified cDNA was performed with T7 RNA polymerase at (42°C, 6 h). The amplified RNA was purified and subjected to a second round of amplification and biotin labeling with Affymetrix's IVT labeling kit according to the manufacturer's directions (Affymetrix, Santa Clara, California). The yield and integrity of the biotin-labeled cRNA were determined with the Nanodrop ND-1000 spectrophotometer and the Agilent 2100 bioanalyzer. Twenty micrograms of biotin-labeled RNA was fragmented to ∼200 bp size by incubation (35 min, 94°C) in fragmentation buffer containing 200 mM Tris-acetate pH 8.2, 500 mM potassium acetate and 500 mM magnesium acetate before hybridization. Fragmented RNA was assessed for relative length on Agilent 2100 bioanalyzer and hybridized to Affymetrix mouse genome 430. 2.0 chips for 16 hours, washed, stained on an Affymetrix fluidics station, and scanned with Affymetrix GeneChip scanner.
Microarray data processing and analysis.
Affymetrix GeneChip operating software version 1.4 was used to calculate signal intensity and the percent present calls on the hybridized Affymetrix chip. The signal-intensity values obtained for probe sets in the microarrays were transformed with an adaptive variance-stabilizing, quantile-normalizing transformation. The transform, termed “S10,” is scaled to match the logarithm transform, base 10. Transformed data from all the chips were subjected to a principal component analysis to detect outliers. Paired t-test was performed to evaluate each probe set. The probability value for differences between the two time points was calculated for each of the 54,675 probe sets. To accommodate statistical concerns resulting from multiple comparisons, fold cutoff filters and false-discovery rate (FDR) analysis filters were applied.Citation70 Two-way hierarchical clustering was used to bring together sets of samples and principal components of the signal intensities on the chips with similar expression patterns using JMP 6.0 (SAS Institute, Cary, NC).
Assay of endogenous Plk1 activity in oocyte lysates.
Ooctye Plk1 activity was assayed as described by Pahlavan et al.Citation52 Briefly, WT and PDE3A-/- oocytes (100 oocytes) were washed in DMEM, collected in 1 µl, lysed in NP-40 buffer (24 µl) (50 mM Hepes, pH 7.4; 1% NP-40; 100 mM NaCl; 25 mM NaF; 25 mM sodium β-glycerophosphate; 1 µg/ml each of soybean trypsin inhibitor, leupeptin, and pepstatin; and 30 µg/ml of DNase I and RNase A), frozen immediately on dry ice, and stored at −80°C. Samples were thawed and clarified by centrifugation (10 min, 10,000 g, 4°C), after which anti-Plk1 antibody (1∼2 µg) (Millipore, Cat#05-844) was added to the supernatants. After incubation (on ice, 2 h), samples were centrifuged (10,000 g, 5 min), and supernatants were removed and incubated with protein G beads (Sigma, Cat#P3296) (1 h, 4°C). Immunocomplexes containing Plk1 were collected by centrifugation (5 min, 2,000 g, 4°C), after which beads were washed once in 1 ml buffer (20 mM Hepes, pH 7.4, 150 mM KCl, 10 mM MgCl2, 1 mM EGTA, 0.5 mM DTT, and 5 mM NaF), and the immunoprecipitated Plk1 (beads) was stored on ice. Plk1 assays were initiated by adding assay buffer (20 µl) (washing buffer supplemented with 10 µM ATP, 4 µCi of [γ-32P]-ATP) and 0.5 mg/ml of dephosphorylated α-casein substrate (Sigma, Cat#C8032) to the immunoprecipitates. After incubation (30 min, 30°C), reactions were terminated by addition of 4x SDS sample buffer. Samples were heated (5 min, 100°C) and subjected to SDS-PAGE. Quantitation of Plk1 activity (phosphorylated α-casein) was analyzed in wet gels using a Phosphor-Imager and the Image- Quant software (Molecular Dynamics, Sunnyvale, CA). After Phosphor-Imager analysis, SDS PAGE gels were electro-transferred to membranes for western blots.
Phosphorylation and activation of recombinant Plk1 (rPlk1) and endogenous Plk1 from Hela cells by purified PKAc.
rPlk1 was phosphorylated in vitro, by incubating (15 min, 30°C) 0.1 µg rPlk1 (Millipore, Cat#14-777) with 20 or 50 units of purified PKAc (Sigma, Cat#P2645) in reaction buffer containing 20 mM MOPS, pH 7.2, 25 mM β-glycerophosphate, 1 mM EGTA, 1 mM Na3VO4, 1 mM DTT, 1 mg/ml BSA, 10 mM MgCl2, 0.1 mM ATP and 4 µCi of [γ-32P]-ATP. Reactions were terminated by adding the PKA inhibitor peptide (Millipore, Cat#12.151) (final concentration 4 µM). Reaction mixtures were divided equally for analysis of rPlk1 activity or phosphorylation of rPlk1, respectively. For rPlk1 activity assays, half volume of reaction mix, containing [γ-32P]-ATP, was incubated (30°C, 20 min) with dephosphorylated α-casein substrate (0.5 mg/ml) (Sigma, Cat#C8032). After addition of 4x SDS sample buffer, samples were heated (100°C, 5 min), and subjected to SDS-PAGE for analysis of phosphorylated α-casein. To assess phosphorylation of rPlk1 by PKAc, anti-Plk1 antibody (1–2 µg) (Millipore, Cat#05-844) was added to the other half volume of reaction mix, which was kept on ice (1 h) and then incubated with protein G beads (4°C, 30 min). After washing 2 times with reaction buffer, 1x SDS sample buffer was added and beads were heated (100°C, 5 min). Eluates were then subjected to SDS-PAGE. Phosphorylation of rPlk1 was quantified using Phosphor-Imager and Image-Quant software (Molecular Dynamics, Sunnyvale, CA).
Endogenous Plk1 was immunoprecipitated from total Hela cell lysates. Briefly, anti-Plk1 antibody (5 µg) was incubated with protein G beads (30 µl) (Invitrogen, Cat#100.03D) for 15 minutes at RT, followed by incubation with total Hela cell lysates (1 mg/IP) for 15 minutes at RT. Beads were collected and washed by ice-cold washing buffer (PBS with 0.02% Tween 20) 4 times, then were used for phosphorylation and activation assays by incubation without and with PKAc as described above.
Statistical analysis.
Data analysis was performed by using SPSS12.0 software package. p < 0.05 was considered to be statistically significant.
Abbreviations
| CAK | = | CDK-activating kinase |
| CDK | = | cell division kinase |
| Cdc2 | = | cell division cycle 2 |
| Cdc5l | = | cell division cycle 5-like |
| Cdc25 | = | cell division cycle 25 |
| Chk | = | checkpoint kinase |
| GV stage | = | germinal vesicle stage |
| GVBD | = | germinal vesicle breakdown |
| LH | = | luteinizing hormone |
| MPF | = | maturation-promoting factor |
| MTOC | = | microtubule organizing center |
| PB stage | = | polar body stage |
| PDE3A | = | phosphodiesterases 3A |
| PKA | = | protein kinase A |
| PKI | = | protein kinase inhibitor |
| Plk1 | = | polo-like kinase 1 |
Figures and Tables
Figure 1 (A) Genotyping. As described in methods, amplification of DNA (100 ng) by PCR yielded fragments of ∼487 bp from PDE3A-/- mice, ∼213 bp from WT mice, and both bands from heterozygous mice. Water was used as a negative control. (B) Western blots of ovarian lysates, performed as described in methods, demonstrated deficiency of immunoreactive PDE3A in PDE3A-/- ovary. (C) Meiotic arrest in PDE3A-/- oocytes. As described in methods, cultured WT oocytes spontaneously resume meiosis in vitro, and, within16 h, extrude the first polar body. PDE3A-/- oocytes remain arrested at GV stage. Oocytes form was captured by a microscopy on objective 20x.
Figure 2 (A and B) PKA activity in cultured oocytes. PKA activity was assayed as described in methods; results are expressed as cpm 32P incorporated into the PKA peptide substrate, Kemptide. (A) In the presence of excess cAMP (2 µM), total PKA activity is similar in lysates from fresh WT and PDE3A-/- oocytes. Basal PKA activity (-cAMP) is significantly increased in PDE3A-/- oocytes, consistent with increased cAMP content in PDE3A-/- oocytes.Citation14 Experiments were repeated three times. Results are presented as m ± SE, n = 3 independent experiments (25 oocytes/assay). (B) Time course of changes in PKA activities: In WT oocytes, PKA activity was reduced for ∼1 h, then increased over the next several hours. In PDE3A-/- oocytes and WT oocytes exposed to cilostamide, PKA activity was elevated during the 5 h incubation. Results are presented as m ± SE, n = 3 independent experiments (25 oocytes/assay). (C) Immunoflurescence of PKA catalytic subunits (PKAc): In freshly isolated WT oocytes, PKAc (green) were detected in the cytoplasm, and some PKAc co-localized with pericentrin (red), presumably on centrosomes. During GVBD, PKAc entered the nucleus and then localized to the spindle apparatus. In PDE3A-/- oocytes, PKAc remained localized in the cytoplasm and on centrosomes, even after incubation for 4 h. n = 3 experiments. Bar, 20 µm.
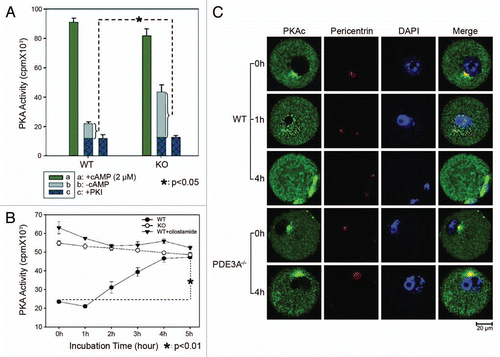
Figure 3 Histone H3 phosphorylation: (A) Oocytes (30 oocytes) were fixed and immunofluoresence performed at antibody dilutions described in methods. In freshly isolated WT oocytes, histone H3 was not phosphorylated at Ser10 (green). In cultured WT oocytes, phosphorylation of histone H3 was initiated within 1 h, and was markedly increased within 4 h, as was histone H3 condensation (an index of chromosomal condensation). In cultured PDE3A-/- oocytes, histone H3 was not phosphorylated at ser10 (green) and chromosome condensation did not occur, even after 18 h.n = 3. (B) At the indicated times, cultured oocytes were harvested, samples (40 WT, 40 PDE3A-/- oocytes) were immediately placed in lysis buffer and lysates were subjected to SDS-PAGE as described in methods. Western Blots demonstrated that after 4 h, histone H3 was phosphorylated at Ser10 in WT oocytes, not in PDE3A-/- oocytes. n = 3 experiments. Bar, 20 µm.
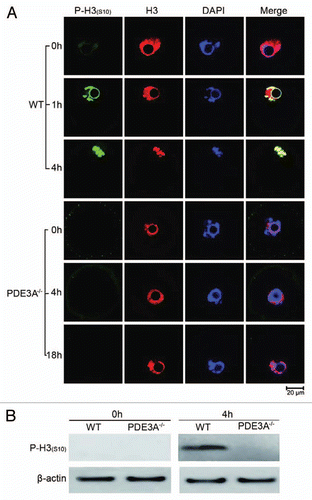
Figure 4 Cdc2 activity and localization: (A) Cultured oocytes were collected at the indicated times, lysates were prepared, and Cdc2 activity was assayed as described in methods. From 1 h to 5 hours, Cdc2 activity significantly increased in cultured WT oocytes, but not in PDE3A-/- oocytes or WT oocytes exposed to cilostamide. Results are expressed as cpm 32P incorporated into the peptide substrate (PKT PKK AKK L) derived from histone H1. Result are presented as m ± SE, n = 3 experiments (25 oocytes/assay at each time point). (B) Western blot. Cultured oocytes (40/time point) were collected at indicated times, and lysates were prepared and subjected to SDS Page/western blots with indicated antibodies as described in methods. Cdc2 was dephosphorylated at T14/Y15 to a much greater extent in WT ooyctes than in PDE3A-/- oocytes; Cdc2 was phosphorylated at Thr161 both in WT and PDE3A-/- oocytes. n = 3 experiments. (C) At the indicated times, cultured oocytes (30 oocytes) were collected and immunoflourescence of Cdc2 was performed as described in methods. In cultured WT oocytes Cdc2 (red) was widely distributed in the cytoplasm and nucleus; during GVBD it localized to the spindle apparatus. Some phospho-Cdc2 [P-Cdc2(T14/Y15)] (green) was localized at MTOCs in fresh WT oocytes, phospho-Cdc2 immunoflouresence was markedly reduced at 4 h and very weak at 18 h. In PDE3A-/- oocytes, phospho-Cdc2 remained in the cytosol, nucleus and at MTOCs. n = 3 experiments. Bar, 20 µm.
![Figure 4 Cdc2 activity and localization: (A) Cultured oocytes were collected at the indicated times, lysates were prepared, and Cdc2 activity was assayed as described in methods. From 1 h to 5 hours, Cdc2 activity significantly increased in cultured WT oocytes, but not in PDE3A-/- oocytes or WT oocytes exposed to cilostamide. Results are expressed as cpm 32P incorporated into the peptide substrate (PKT PKK AKK L) derived from histone H1. Result are presented as m ± SE, n = 3 experiments (25 oocytes/assay at each time point). (B) Western blot. Cultured oocytes (40/time point) were collected at indicated times, and lysates were prepared and subjected to SDS Page/western blots with indicated antibodies as described in methods. Cdc2 was dephosphorylated at T14/Y15 to a much greater extent in WT ooyctes than in PDE3A-/- oocytes; Cdc2 was phosphorylated at Thr161 both in WT and PDE3A-/- oocytes. n = 3 experiments. (C) At the indicated times, cultured oocytes (30 oocytes) were collected and immunoflourescence of Cdc2 was performed as described in methods. In cultured WT oocytes Cdc2 (red) was widely distributed in the cytoplasm and nucleus; during GVBD it localized to the spindle apparatus. Some phospho-Cdc2 [P-Cdc2(T14/Y15)] (green) was localized at MTOCs in fresh WT oocytes, phospho-Cdc2 immunoflouresence was markedly reduced at 4 h and very weak at 18 h. In PDE3A-/- oocytes, phospho-Cdc2 remained in the cytosol, nucleus and at MTOCs. n = 3 experiments. Bar, 20 µm.](/cms/asset/1988a3a3-b403-40ae-9aea-463481446097/kccy_a_10914090_f0004.gif)
Figure 5 (A) Immunoflourescence of Cyclin B1 in cultured oocytes (30 oocytes each time point) was performed as described in methods. In fresh WT oocytes, Cyclin B1 signal (green) was primarily in the cytoplasm, with some localization at MTOCs. Within 1 h, Cyclin B1 immunofluoresence increased in nucleus, and within 4 h, localized to the spindle apparatus and centrosomes. In cultured PDE3A-/- oocytes, Cyclin B1 localization did not change, even after 4 h incubation. n = 3 experiments. (B) Western blots. At the indicated times, cultured oocytes (40 oocytes/time point) were collected, and lysates were prepared and subjected to SDS PAGE/western blots as described in methods. Immunoreactive Cyclin B1 seems to transiently increase and then decrease in cultured PDE3A-/- oocytes. n = 3 experiments. Bar, 20 µm.
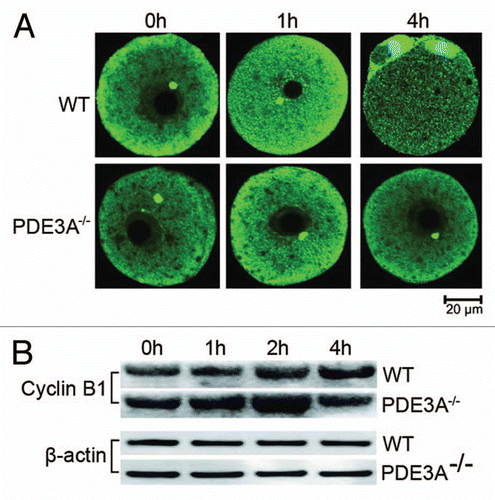
Figure 6 (A) Immunoflourescence of Cdc25B in cultured oocytes. At indicated times, cultured oocytes (30 oocytes each time point) were collected, fixed and immunofluorescence of Cdc25B (green) assessed as described in methods. In freshly prepared WT and PDE3A-/- oocytes, immunoreactive Cdc25B (Green) was relatively more highly expressed in nucleus than in cytoplasm. Within 1 h, nuclear Cdc25B was decreased in cultured WT, not PDE3A-/-, oocytes. After 4 h, Cdc25B immunofluoresence was markedly decreased in GVBD WT oocytes as compared to cultured PDE3A-/- oocytes, in which nuclear Cdc25B was relatively stable during the 4 h incubation. In the 4 h WT panel, the chromatin at the bottom left is most likely from accompanying cumulus cells. n = 3 experiments. (B) Western blots of phospho-Cdc25B. At indicated times, cultured oocytes were collected (40 oocytes/time point), and lysates were prepared and subjected to SDS PAGE/western blots, using anti-phospho-Cdc25B (Ser323), as described in methods. Cdc25B was dephosphorylated at Ser323 within 1 h in cultured WT oocytes, but not in PDE3A-/- oocytes. n = 3 experiments. Bar, 20 µm.
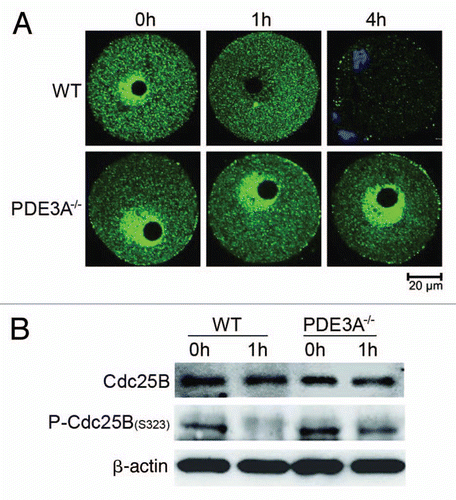
Figure 7 Wee1 and Check kinase activities in cultured oocytes. At indicated times, cultured WT and PDE3A-/- oocytes were collected (100 oocytes/time point), lysates prepared, and enzymatic assays performed as described in methods. Results are presented as m ± SE, n = 3 experiments. (A) Time course of Wee1 activity: Wee1 activity (expressed as the color quantitated by spectrophotometry at 450 nm) decreased in cultured PDE3A-/- and WT oocytes, and WT oocytes exposed to cilostamide. (B) Check kinase activity: There are no significant differences in checkpoint kinase activity (expressed as the color quantitated by spectrophotometry at 450 nm) between cultured WT and PDE3A-/- oocytes.
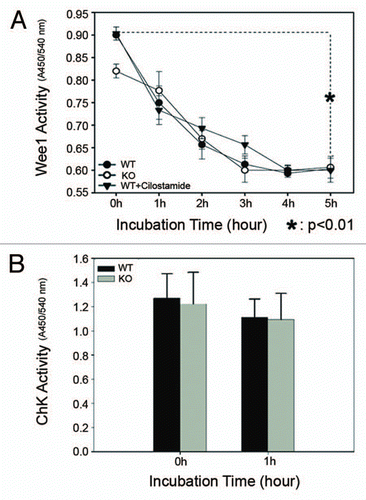
Figure 8 Western blot of key proteins for G2/M transition. As described in methods, lysates from freshly prepared WT and PDE3A-/- oocytes (40 oocytes) were subjected to SDS-PAGE/western Blot. Membranes were incubated with indicated primary antibodies at 4°C overnight, followed with second antibody for 1 h at room temperature.
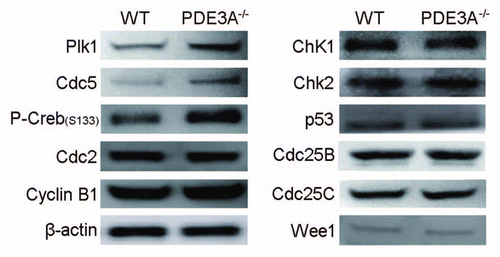
Figure 9 (A) Plk1 activity in cultured oocytes. Cultured oocytes (100 oocytes per time point) were collected at the indicated times to assess Plk1 activity. Some WT oocytes (100 oocytes) were treated with cilostamide (10 µM) for 4 h, and some PDE3A-/- oocytes (100 oocytes) were treated with the PKA inhibitor, Rp-cAMP (5 mM), for 2 h and then incubated in the absence of Rp-cAMP for 2 h before Plk1 assay. Plk1 was immunprecipitated from oocyte lysates. Plk1 activity was assayed by incubation of immunoprecipitated Plk1 with 32P-ATP and α-casein substrate as described in methods. After Phosphor Imager analysis of wet gels to measure 32P-labelled α-casein, SDS PAGE gels were electro-transferred to membranes for western blots for detection of immunoreactive oocyte Plk1. n = 3 experiments. (B) Phosphorylation/inactivation of rPlk1 by purified PKAc in vitro. As described in methods, rPlk1 was incubated with indicated units of purified PKAc and 32P-ATP at 30°C for 15 min; PKI (4 µM) was added to terminate the reaction. To half volume of the mixture, dephosphorylated α-casein substrate (0.5 mg/ml) was added, to assess Plk1 activity i.e., Plk1-induced phosphorylation of α-casein. The other half volume was used for Plk1 immunoprecipitation to check PKAc-induced phosphorylation of rPlk1. Results showed that purified PKAc phosphorylated rPlk1 in vitro and inhibited its activity. n = 5 experiments. (C) PDE3A co-immunoprecipitates with Plk1 in mice ovary and Hela cells. As described in methods, total lysates were prepared from WT mouse ovaries (to increase the amount of material available) (0.6 mg/IP) and Hela cells (2.0 mg/IP) and incubated with anti-PDE3A or anti-Plk1 antibodies or non-immine IgG. Immunoprecipitates were subjected to SDS PAGE and western blots, using the indicated primary antibodies. PDE3A and Plk1 co-immunoprecipitated in lysates from mouse ovary and Hela cells. n = 3 experiments. (D) PKA inhibited endogenous Plk1 activity in vitro and Plk1 interacted with PDE3A-PKAc macromolecular complex in Hela cells. (Upper part) Endogenous Plk1 was immunoprecipitated from Hela cells (1 mg/IP), followed by incubation of immunoprecipitated Plk1 (beads) with indicated units of purified PKAc and 32P-ATP at 30°C for 15 min; PKI (4 µM) was added to terminate the reaction. PKAc-induced phosphorylation of Hela cell Plk1 and inhibition of Plk1-induced phosphorylation of “-casein substrate were analyzed as described in methods and in legend to (B). (Lower part): Hela cells were homogenized in ice-cold buffer A [50 mM Hepes, 1 mM EDTA, 10 mM pyrophosphate, 5 mM MgCl2, 5 mM NaF, 100 mM NaCl, 0.1 µM okadaic acid, phosphatase inhibitor cocktail (Calbiochem) and Roche protease inhibitor cocktail (pH 7.5)] with dounce homogenizer (on ice, 20 strokes), sonicated (on ice, 20 pulses, 40% duty cycle, output scale 4), and solubilized with 1% NP-40 (Calbiochem) (1% final). Samples were cleared by incubation (1 h) with 5 µg non-immune IgG, and then with Dynabeads protein G (Invitrogen, Cat# 100.04D) for 30 min before centrifugation (2,800 g, 4°C, 5 min). Cleared lysates from Hela cells (2 mg/IP) were incubated (overnight, 4°C) with mouse anti-Plk1 (10 µg/IP) (Millipore, Cat# 05-844) and non-immune mouse IgG (10 µg/IP), followed by incubation (1 h) with fresh Dynabeads protein G as described in methods. Immunoblot data indicated that Plk1 co-immunoprecipitated with PDE3A, PKA-C, PP2A and Cdc25C. n = 3 experiments. (E) Subcelluar localization of Plk1 and PDE3A in WT and PDE3A-/- oocytes. As described in methods, at the indicated times, cultured oocytes were collected, fixed, and assessed for immunofluorescence of PDE3A and Plk1. In freshly prepared WT oocytes, Plk1 was detected in the cytoplasm, with some localization at MTOCs. Within 1 hour, Plk1 migrated into the nucleus, and at GVBD, it was detected at MTOCs and kinetochores. In cultured WT oocytes PDE3A was partly co-localized with Plk1 at the spindle apparatus. In cultured PDE3A-/- oocytes, even after 4 hours, Plk1 immunofluorescence was only detected in the cytoplasm, with some localization at MTOCs, but not in the nucleus. No PDE3A signal was detected in PDE3A-/- oocytes. n = 3 experiments. Bar, 20 µm.
![Figure 9 (A) Plk1 activity in cultured oocytes. Cultured oocytes (100 oocytes per time point) were collected at the indicated times to assess Plk1 activity. Some WT oocytes (100 oocytes) were treated with cilostamide (10 µM) for 4 h, and some PDE3A-/- oocytes (100 oocytes) were treated with the PKA inhibitor, Rp-cAMP (5 mM), for 2 h and then incubated in the absence of Rp-cAMP for 2 h before Plk1 assay. Plk1 was immunprecipitated from oocyte lysates. Plk1 activity was assayed by incubation of immunoprecipitated Plk1 with 32P-ATP and α-casein substrate as described in methods. After Phosphor Imager analysis of wet gels to measure 32P-labelled α-casein, SDS PAGE gels were electro-transferred to membranes for western blots for detection of immunoreactive oocyte Plk1. n = 3 experiments. (B) Phosphorylation/inactivation of rPlk1 by purified PKAc in vitro. As described in methods, rPlk1 was incubated with indicated units of purified PKAc and 32P-ATP at 30°C for 15 min; PKI (4 µM) was added to terminate the reaction. To half volume of the mixture, dephosphorylated α-casein substrate (0.5 mg/ml) was added, to assess Plk1 activity i.e., Plk1-induced phosphorylation of α-casein. The other half volume was used for Plk1 immunoprecipitation to check PKAc-induced phosphorylation of rPlk1. Results showed that purified PKAc phosphorylated rPlk1 in vitro and inhibited its activity. n = 5 experiments. (C) PDE3A co-immunoprecipitates with Plk1 in mice ovary and Hela cells. As described in methods, total lysates were prepared from WT mouse ovaries (to increase the amount of material available) (0.6 mg/IP) and Hela cells (2.0 mg/IP) and incubated with anti-PDE3A or anti-Plk1 antibodies or non-immine IgG. Immunoprecipitates were subjected to SDS PAGE and western blots, using the indicated primary antibodies. PDE3A and Plk1 co-immunoprecipitated in lysates from mouse ovary and Hela cells. n = 3 experiments. (D) PKA inhibited endogenous Plk1 activity in vitro and Plk1 interacted with PDE3A-PKAc macromolecular complex in Hela cells. (Upper part) Endogenous Plk1 was immunoprecipitated from Hela cells (1 mg/IP), followed by incubation of immunoprecipitated Plk1 (beads) with indicated units of purified PKAc and 32P-ATP at 30°C for 15 min; PKI (4 µM) was added to terminate the reaction. PKAc-induced phosphorylation of Hela cell Plk1 and inhibition of Plk1-induced phosphorylation of “-casein substrate were analyzed as described in methods and in legend to (B). (Lower part): Hela cells were homogenized in ice-cold buffer A [50 mM Hepes, 1 mM EDTA, 10 mM pyrophosphate, 5 mM MgCl2, 5 mM NaF, 100 mM NaCl, 0.1 µM okadaic acid, phosphatase inhibitor cocktail (Calbiochem) and Roche protease inhibitor cocktail (pH 7.5)] with dounce homogenizer (on ice, 20 strokes), sonicated (on ice, 20 pulses, 40% duty cycle, output scale 4), and solubilized with 1% NP-40 (Calbiochem) (1% final). Samples were cleared by incubation (1 h) with 5 µg non-immune IgG, and then with Dynabeads protein G (Invitrogen, Cat# 100.04D) for 30 min before centrifugation (2,800 g, 4°C, 5 min). Cleared lysates from Hela cells (2 mg/IP) were incubated (overnight, 4°C) with mouse anti-Plk1 (10 µg/IP) (Millipore, Cat# 05-844) and non-immune mouse IgG (10 µg/IP), followed by incubation (1 h) with fresh Dynabeads protein G as described in methods. Immunoblot data indicated that Plk1 co-immunoprecipitated with PDE3A, PKA-C, PP2A and Cdc25C. n = 3 experiments. (E) Subcelluar localization of Plk1 and PDE3A in WT and PDE3A-/- oocytes. As described in methods, at the indicated times, cultured oocytes were collected, fixed, and assessed for immunofluorescence of PDE3A and Plk1. In freshly prepared WT oocytes, Plk1 was detected in the cytoplasm, with some localization at MTOCs. Within 1 hour, Plk1 migrated into the nucleus, and at GVBD, it was detected at MTOCs and kinetochores. In cultured WT oocytes PDE3A was partly co-localized with Plk1 at the spindle apparatus. In cultured PDE3A-/- oocytes, even after 4 hours, Plk1 immunofluorescence was only detected in the cytoplasm, with some localization at MTOCs, but not in the nucleus. No PDE3A signal was detected in PDE3A-/- oocytes. n = 3 experiments. Bar, 20 µm.](/cms/asset/0ddbd1c2-c1ea-481c-a26b-f49058ef6173/kccy_a_10914090_f0009.gif)
Figure 10 Proposed effects of PDE3A on regulation of cAMP/PKA signaling during meiotic progression in mice oocytes. In PDE3A-/- oocytes, activated PKA phosphorylates and inhibits Cdc25B and Plk1 directly, but phosphorylates/activates Wee1 kinase, and Myt1 is not subject to inhibition by Plk1. The integrated effect of these PKA-induced phosphorylations is inactivation of Cdc2 and maintainence of G2/M meiotic arrest. In WT oocytes, during GVBD, PKA is translocalized to the nucleus, where it may phosphorylate histone H3 to initiate DNA condensation; PKA-translocalization, histone H3 phosphorylation, and chromosome condensation does not occur in cultured PDE3A-/- oocytes. This scheme does not include possible “feed-forward amplification” of meiotic progression by activated MPF, i.e., activated Cdc2 activates Cdc25B and Plk1 (which inhibits Myt1), and inhibits Wee1 kinase, allowing further activation of MPF.
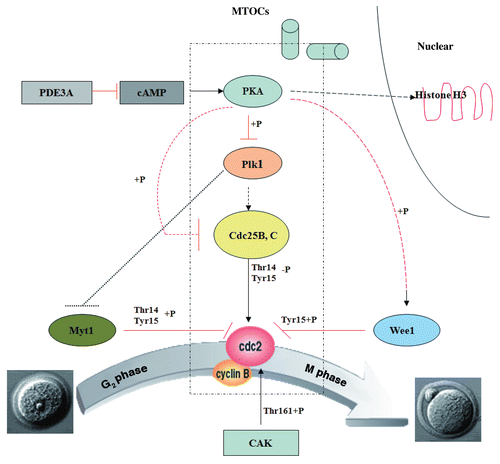
Table 1 Affymetrix microarray of key genes for G2/M transition in WT and PDE3A-/- oocytes
Acknowledgements
This research was funded by the NHLBI Intramural Research Program.
References
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: Essential components in cyclic nucleotide signaling. Annu Rev Biochem 2007; 76:481 - 511
- Thompson PE, Manganiello V, Degerman E. Re-discovering PDE3 inhibitors—new opportunities for a long neglected target. Curr Top Med Chem 2007; 7:421 - 436
- Sun QY, Miao YL, Schatten H. Towards a new understanding on the regulation of mammalian oocyte meiosis resumption. Cell Cycle 2009; 8:2741 - 2747
- Conti M. Signaling networks in somatic cells and oocytes activated during ovulation. Ann Endocrinol (Paris) 2010; 71:189 - 190
- Dekel N. Cellular, biochemical and molecular mechanisms regulating oocyte maturation. Mol Cell Endocrinol 2005; 234:19 - 25
- Han SJ, Conti M. New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate. Cell Cycle 2006; 5:227 - 231
- Duckworth BC, Weaver JS, Ruderman JV. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc Natl Acad Sci USA 2002; 99:16794 - 16799
- Zhang Y, Zhang Z, Xu XY, Li XS, Yu M, Yu AM, et al. Protein kinase A modulates Cdc25B activity during meiotic resumption of mouse oocytes. Dev Dyn 2008; 237:3777 - 3786
- Pirino G, Wescott MP, Donovan PJ. Protein kinase A regulates resumption of meiosis by phosphorylation of Cdc25B in mammalian oocytes. Cell Cycle 2009; 8:665 - 670
- Han SJ, Chen R, Paronetto MP, Conti M. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol 2005; 15:1670 - 1676
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, et al. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 2009; 136:1869 - 1878
- Vaccari S, Weeks JL 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod 2009; 81:595 - 604
- Shitsukawa K, Andersen CB, Richard FJ, Horner AK, Wiersma A, van Duin M, et al. Cloning and characterization of the cyclic guanosine monophosphate-inhibited phosphodiesterase PDE3A expressed in mouse oocyte. Biol Reprod 2001; 65:188 - 196
- Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, et al. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest 2004; 114:196 - 205
- Richard FJ, Tsafriri A, Conti M. Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod 2001; 65:1444 - 1451
- Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell Cycle 1991; 64:1111 - 1122
- Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science 1992; 257:1955 - 1957
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: A membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 1995; 270:86 - 90
- Wells NJ, Watanabe N, Tokusumi T, Jiang W, Verdecia MA, Hunter T. The C-terminal domain of the Cdc2 inhibitory kinase Myt1 interacts with Cdc2 complexes and is required for inhibition of G(2)/M progression. J Cell Sci 1999; 112:3361 - 3371
- Fesquet D, Labbé JC, Derancourt J, Capony JP, Galas S, Girard F, et al. The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin-dependent kinases (CDKs) through phosphorylation of Thr161 and its homologues. EMBO J 1993; 12:3111 - 3121
- Solomon MJ, Harper JW, Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J 1993; 12:3133 - 3142
- Karlsson-Rosenthal C, Millar JB. Cdc25: Mechanisms of checkpoint inhibition and recovery. Trends Cell Biol 2006; 16:285 - 292
- Boutros R, Dozier C, Ducommun B. The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol 2006; 18:185 - 191
- Lincoln AJ, Wickramasinghe D, Stein P, Schultz RM, Palko ME, De Miguel MP, et al. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat Genet 2002; 30:446 - 449
- Solc P, Saskova A, Baran V, Kubelka M, Schultz RM, Motlik J. CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev Biol 2008; 317:260 - 269
- Oh JS, Han SJ, Conti M. Wee1B, Myt1 and Cdc25 function in distinct compartments of the mouse oocyte to control meiotic resumption. J Cell Biol 2010; 188:199 - 207
- Archambault V, Glover DM. Polo-like kinases: Conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol 2009; 10:265 - 275
- Barr FA, Silljé HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol 2004; 5:429 - 440
- Kishimoto T. Cell cycle control during meiotic maturation. Curr Opin Cell Biol 2003; 15:654 - 663
- Lobjois V, Jullien D, Bouché JP, Ducommun B. The polo-like kinase 1 regulates CDC25B-dependent mitosis entry. Biochim Biophys Acta 2009; 1793:462 - 468
- Roshak AK, Capper EA, Imburgia C, Fornwald J, Scott G, Marshall LA. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell Signal 2000; 12:405 - 411
- Qian YW, Erikson E, Taieb FE, Maller JL. The polo-like kinase Plx1 is required for activation of the phosphatase Cdc25C and cyclin B-Cdc2 in Xenopus oocytes. Mol Biol Cell 2001; 12:1791 - 1799
- Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science 1996; 273:1377 - 1380
- Lindqvist A, Rodríguez-Bravo V, Medema RH. The decision to enter mitosis: Feedback and redundancy in the mitotic entry network. J Cell Biol 2009; 185:193 - 202
- Abrieu A, Brassac T, Galas S, Fisher D, Labbé JC, Dorée M. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J Cell Sci 1998:1751 - 1757
- Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J Biol Chem 2003; 278:25277 - 25280
- Inoue D, Sagata N. The Polo-like kinase Plx1 interacts with and inhibits Myt1 after fertilization of Xenopus eggs. EMBO J 2005; 24:1057 - 1067
- Toyoshima-Morimoto F, Taniguchi E, Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep 2002; 3:341 - 348
- Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A, Nishida E. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 2001; 410:215 - 220
- Wiersma A, Hirsch B, Tsafriri A, Hanssen RG, Van de Kant M, Kloosterboer HJ, et al. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J Clin Invest 1998; 102:532 - 537
- Gurley LR, D'Anna JA, Barham SS, Deaven LL, Tobey RA. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem 1978; 84:1 - 15
- Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD. Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci USA 1998; 95:7480 - 7484
- Van Hooser A, Goodrich DW, Allis CD, Brinkley BR, Mancini MA. Histone H3 phosphorylation is required for the initiation, but not maintenance, of mammalian chromosome condensation. J Cell Sci 1998; 111:3497 - 3506
- DeManno DA, Cottom JE, Kline MP, Peters CA, Maizels ET, Hunzicker-Dunn M. Follicle-stimulating hormone promotes histone H3 phosphorylation on serine-10. Mol Endocrinol 1999; 13:91 - 105
- Huo LJ, Yu LZ, Liang CG, Fan HY, Chen DY, Sun QY. Cell cycle-dependent subcellular localization of cyclin B1, phosphorylated cyclin B1 and p34cdc2 during oocyte meiotic maturation and fertilization in mouse. Zygote 2005; 13:45 - 53
- Li J, Meyer AN, Donoghue DJ. Nuclear localization of cyclin B1 mediates its biological activity and is regulated by phosphorylation. Proc Natl Acad Sci USA 1997; 94:502 - 507
- Gershon E, Galiani D, Dekel N. Cytoplasmic polyadenylation controls cdc25B mRNA translation in rat oocytes resuming meiosis. Reproduction 2006; 132:21 - 31
- Gabrielli BG, De Souza CP, Tonks ID, Clark JM, Hayward NK, Ellem KA. Cytoplasmic accumulation of cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J Cell Sci 1996; 109:1081 - 1093
- Krömer A, Mailand N, Lukas C, Syljuåsen RG, Wilkinson CJ, Nigg EA, et al. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat Cell Biol 2004; 6:884 - 891
- Sivasubramaniam S, Sun X, Pan YR, Wang S, Lee EY. Cep164 is a mediator protein required for the maintenance of genomic stability through modulation of MDC1, RPA and CHK1. Genes Dev 2008; 22:587 - 600
- Rivers DM, Moreno S, Abraham M, Ahringer J. PAR proteins direct asymmetry of the cell cycle regulators Polo-like kinase and Cdc25. J Cell Biol 2008; 180:877 - 885
- Pahlavan G, Polanski Z, Kalab P, Golsteyn R, Nigg EA, Maro B. Characterization of polo-like kinase 1 during meiotic maturation of the mouse oocyte. Dev Biol 2000; 220:392 - 400
- Takizawa CG, Morgan DO. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr Opin Cell Biol 2000; 12:658 - 665
- Smits VA, Medema RH. Checking out the G(2)/M transition. Biochim Biophys Acta 2001; 1519:1 - 12
- Wang J, Cao WL, Liu XJ. Protein kinase A(PKA)-restrictive and PKA-permissive phases of oocyte maturation. Cell Cycle 2006; 5:213 - 217
- Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol 2003; 5:143 - 148
- Bailly E, Pines J, Hunter T, Bornens M. Cytoplasmic accumulation of cyclin B1 in human cells: association with a detergent-resistant compartment and with the centrosome. J Cell Sci 1992; 101:529 - 545
- Tsvetkov L, Xu X, Li J, Stern DF. Polo-like kinase 1 and Chk2 interact and co-localize to centrosomes and the midbody. J Biol Chem 2003; 278:8468 - 8475
- Matyakhina L, Lenherr SM, Stratakis CA. Protein kinase A and chromosomal stability. Ann NY Acad Sci 2002; 968:148 - 157
- Carlson CR, Witczak O, Vossebein L, Labbé JC, Skålhegg BS, Keryer G, et al. CDK1-mediated phosphorylation of the RIIalpha regulatory subunit of PKA works as a molecular switch that promotes dissociation of RIIalpha from centrosomes at mitosis. J Cell Sci 2001; 3243 - 3254
- Kovo M, Schillace RV, Galiani D, Josefsberg LB, Carr DW, Dekel N. Expression and modification of PKA and AKAPs during meiosis in rat oocytes. Mol Cell Endocrinol 2002; 192:105 - 113
- Brown RL, Ord T, Moss SB, Williams CJ. A-kinase anchor proteins as potential regulators of protein kinase A function in oocytes. Biol Reprod 2002; 67:981 - 987
- Kelm O, Wind M, Lehmann WD, Nigg EA. Cell cycle-regulated phosphorylation of the Xenopus polo-like kinase Plx1. J Biol Chem 2002; 277:25247 - 25256
- Okano-Uchida T, Okumura E, Iwashita M, Yoshida H, Tachibana K, Kishimoto T. Distinct regulators for Plk1 activation in starfish meiotic and early embryonic cycles. EMBO J 2003; 22:5633 - 5642
- Fu Z, Malureanu L, Huang J, Wang W, Li H, van Deursen JM, et al. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol 2008; 10:1076 - 1082
- Xiong B, Sun SC, Lin SL, Li M, Xu BZ, OuYang YC, et al. Involvement of Polo-like kinase 1 in MEK1/2-regulated spindle formation during mouse oocyte meiosis. Cell Cycle 2008; 15:1804 - 1809
- Golsteyn RM, Mundt KE, Fry AM, Nigg EA. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J Cell Biol 1995; 129:1617 - 1628
- Tong C, Fan HY, Lian L, Li SW, Chen DY, Schatten H, et al. Polo-like kinase-1 is a pivotal regulator of microtubule assembly during mouse oocyte meiotic maturation, fertilization, and early embryonic mitosis. Biol Reprod 2002; 67:546 - 554
- Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol 1996; 135:1701 - 1713
- Klipper-Aurbach Y, Wasserman M, Braunspiegel-Weintrob N, Borstein D, Peleg S, Assa S, et al. Mathematical formulae for the prediction of the residual beta cell function during the first two years of disease in children and adolescents with insulin-dependent diabetes mellitus. Med Hypotheses 1995; 45:486 - 490
- Bonnet J, Coopman P, Morris MC. Characterization of centrosomal localization and dynamics of Cdc25C phosphatase in mitosis. Cell Cycle 2008; 7:1991 - 1998
- Krämer A, Lukas J, Bartek J. Checking out the centrosome. Cell Cycle 2004; 3:1390 - 1393
- Bailly E, Doree M, Nurse P, Bormens M. p34cdc2 is located in both nucleus and cytoplasm; patr is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J 1989; 8:3985 - 3995
- Ookata K, Hisanaga S, Okano T, Tachibana K, Kishimoto T. Relocation and distinct subcellular localization of p34cdc2-cyclin B complex at meiosis reinitiation in starfish oocytes. EMBO J 1992; 11:1763 - 1772
- Andreassen PR, Margolis RL. Microtubule dependency of p34cdc2 inactivation and mitotic exit in mammalian cells. J Cell Biol 1994; 127:789 - 802
- Morisaki T, Hirota T, Iida S, Marumoto T, Hara T, Nishiyama Y, et al. WARTS tumor suppressor is phosphorylated by Cdc2/cyclin B at spindle poles during mitosis. FEBS Lett 2002; 529:319 - 324
- Wu Q, Guo Y, Yamada A, Perry JA, Wang MZ, Araki M, et al. A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr Biol 2007; 17:213 - 224
- Chung E, Chen RH. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat Cell Biol 2003; 5:748 - 753
- Wong OK, Fang G. Cdk1 phosphorylation of BubR1 controls spindle checkpoint arrest and Plk1-mediated formation of the 3F3/2 epitope. J Cell Biol 2007; 179:611 - 617
- Athar M, Kim AL, Ahmad N, Mukhtar H, Gautier J, Bickers DR. Mechanism of ultraviolet B-induced cell cycle arrest in G2/M phase in immortalized skin keratinocytes with defective p53. Biochem Biophys Res Commun 2000; 277:107 - 111
- Narayanan PK, Rudnick JM, Walthers EA, Crissman HA. Modulation in cell cycle and cyclin B1 expression in irradiated HeLa cells and normal human skin fibroblasts treated with staurosporine and caffeine. Exp Cell Res 1997; 233:118 - 127
- Nascimento R, Parkhouse RM. Murine gammaherpesvirus 68 ORF20 induces cell cycle arrest in G2 by inhibiting the Cdc2-cyclin B complex. J Gen Virol 2007; 88:1446 - 1453